Summary
This cross-sectional study assessed the prevalence of Achilles tendinopathy symptoms and ultrasound (US) abnormalities in male and female ice skaters, and compared this to age-matched controls. The 20 skaters of mean (sd) age 17.3 (7.9) were recruited from British figure skating clubs. The 17 non-skaters of mean age 18.0 (3.7) were recruited from a secondary school and university. Each group had 12 females. All participants completed a questionnaire, and Achilles tendons were ultrasound-scanned for thickening, hypoechoic areas, paratenon blurring and neovascularization. Skaters experienced significantly more lifetime symptoms (p=0.012) than the control group but there were no differences in present symptoms. Mid-tendon longitudinal thickness and the coefficient of variation (CoV) for longitudinal tendon thickness were significantly greater in the skaters (p=0.001 and p=0.017 respectively). No other ultrasound abnormalities were detected in either group. Figure skaters may be at a greater risk of Achilles tendon problems than the general population and have adaptive changes in their tendons.
Keywords: Achilles, skating, sonography, tendinopathy, ultrasonography
Introduction
Over the past two decades training time and intensity of figure skating have increased in response to rising expectations1, with a likely increase in repetitive forces propagated through the lower limb2. If such forces repeatedly stress the Achilles tendon fibres beyond their physiological limit, an increased risk of Achilles tendinopathy may ensue3,4. Furthermore, although the foot is somewhat protected by the skating boot, it is maintained in slight plantarflexion. This may cause tightening of the calf muscles, which may be linked to Achilles tendinopathy4. Achilles tendinopathy produces pain, swelling, stiffness and tenderness in and around the tendon5. It is often associated with impaired function that may prevent the athlete from pursuing their sport6. Currently no research has directly investigated the prevalence of Achilles tendinopathy symptoms in figure skaters.
Ultrasonography (US) is a valid and reliable indicator of Achilles tendinopathy7. General increases in tendon thickness may be a feature of tendinopathy 8,9 and hypoechoic areas, focal thickening, blurred margins and increased blood flow 10,11 are considered a frequent feature. To our knowledge no studies have assessed the Achilles tendons of skaters for US abnormalities. Such assessment may have particular importance because sonographic markers of tendinopathy are present in asymptomatic tendons in other sports12,13, and these tendons may be at higher risk of becoming symptomatic14,15. Detection of such markers in asymptomatic skaters might therefore suggest further longitudinal work evaluating whether these markers can predict future symptoms as well as informing clinicians looking after such athletes.
The primary hypothesis of this study was that the prevalence of symptoms and the ultrasound characteristics of the Achilles tendons in a group of figure skaters would differ from age-matched controls. The secondary hypothesis was that ultrasound abnormalities indicative of pathology would occur without the presence of symptoms in some skaters.
Materials and methods
Subjects
Male and female subjects were recruited, within the age range of 12–45. The study group was recruited from British figure skating clubs by contacting coaches and club secretaries. Skaters were approached during training sessions on the day of data collection. The control group was recruited from a secondary school and a university in a similar manner. Individuals under the age of 12 were excluded from both groups, and subjects with figure skating experience were excluded from the control group.
Procedure
Height, weight, age, gender and leg dominance data were collected. The dominant leg was defined as the leg the subject predominantly jumped with. The study group completed a questionnaire about skating discipline and level of competition, average training hours per week, and the number of years that they had been training at that intensity. Achilles tendinopathy symptoms, current or previous, and the amount of training missed as a consequence were also recorded (Appendix 1). The control group filled in a similar questionnaire, which was modified to exclude specific figure skating questions, asking about average hours of sport or exercise per week instead of training duration. The primary investigator was blinded to the symptomatic status of the subjects; a second investigator collected the anthropometric data and questionnaires and the primary investigator did not have access to these data until after analysis of the US scans.
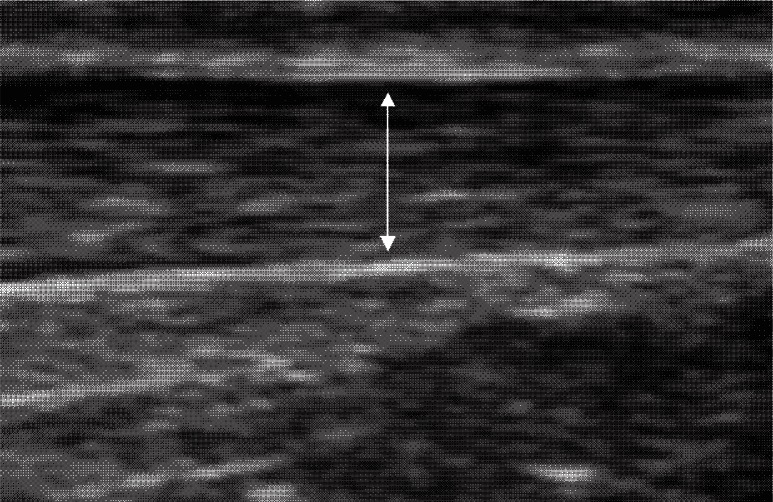
US scans were performed with a high-resolution 3–12MHz linear transducer (Voluson-i, GE medical systems, UK) by the primary investigator, who had attended a musculoskeletal ultrasound course and gained further experience under the supervision of a musculoskeletal consultant radiologist. The machine settings were standardised prior to examination for optimal visualisation of the Achilles tendon. The subjects lay prone on an examination couch, with their feet over the end of the couch. To standardise the measurements, ankles were fixed at 90° with a device constructed for this purpose. Care was taken to keep the transducer perpendicular to the tendon fibres at all times to reduce the anisotropy effect10. Grey-scale images were obtained at three points along both right and left tendons in the longitudinal plane. These points were the insertion at the calcaneus, the musculotendinous junction (MTJ) between tendon and soleus muscle, and midway between these points (mid-tendon). These images were recorded and analysed offline using Image J 1.38x software (National Institutes of Health, USA). The tendon thickness was measured at the three points along each tendon on the longitudinal images (Fig. 1). The mean and coefficient of variation (CoV) of these three measurements were calculated for each tendon. The presence or otherwise of degenerative markers - hypoechoic areas, focal thickening and paratenon blurring - were assessed throughout the tendon length in the longitudinal view. The presence or otherwise of tendon vascularity was examined longitudinally in real time with colour Doppler.
Figure 1.
Measurements in the longitudinal plane.
Reliability study
Intra-observer reliability was assessed. The primary investigator scanned eight Achilles tendons using the protocol described above. The same tendons were then rescanned later the same day. All images were recorded, and analysed by measurement of tendon thickness at the 3 points. The test-retest reliability for each of the three measurement points was calculated using Intraclass Correlation Coefficients (ICCs) type 3,1 and limits of agreement (LOA)16,17.
Data analysis
Sample size calculations, using a power of 80% and an alpha of 5%, and Achilles mid-tendon thickness group difference and variance data derived from a similar study on gymnasts18, indicated that 32 tendons were needed in each group.
Focal thickening was quantified by calculating the co-efficient of variation (CoV) of tendon thickness across the three points, using the formula: CoV = sd of the 3 measures/mean of the 3 measures. Subjects were classified as having US abnormalities by having at least one sign of neovascularisation, hypoechoic areas, or paratenon blurring at any region along the tendon.
SPSS version 15.0 (SPSS Inc, Chicago, USA) was used to analyse the data. Continuous data were tested for normality by inspecting their distribution in histograms. The normally distributed age, height and weight variables were tested for group differences with independent t tests. As the other continuous data were generally skewed, all tendon thickness data were presented as medians and interquartile ranges, and group differences were tested for significance with nonparametric Mann-Whitney U tests. Mann-Whitney U tests were also used to compare ultrasound tendon thickness findings between symptomatic and non symptomatic groups within skaters and controls. Pearson’s Chi-Square tests were used to compare present and lifetime history symptom status between the groups and to compare their gender composition. For all calculations, 95% confidence intervals were used, and p values<0.05 were considered statistically significant.
Ethical Considerations
The study received local ethical approval for human studies from Queen Mary University of London research ethics committee. Informed consent was obtained from all subjects.
Results
Subject data:
Twenty figure skaters were recruited; 12 female and 8 male. Sixteen skaters competed in singles free skating, 3 in pairs, 9 in ice dance and 8 were part of a synchronised skating team. There was some overlap between these categories, with some skaters participating in more than one discipline. The level at which they competed ranged from novice to elite. They trained for a mean (sd) of 11.5 (6.0) hours a week, including both on- and office training sessions, and had been training at this intensity for an average of 5.4 years. In addition, most skaters took part in other sports or exercise, either at school or in their spare time.
Seventeen active controls were recruited: 12 female and 5 male. Eight controls were from a secondary school and 9 were from a university. The mean (sd) of sport or exercise the controls did per week was 5.3 (2.7) hours, which was significantly less (p<0.001) than the skaters.
There was no significant difference in gender between the two groups (chi square=0.904, p=0.34), nor were there significant differences in age, height or weight (Tab. 1).
Table 1.
Characteristics of the groups.
| Characteristic | Figure skaters [mean (SD)] | Controls [mean (SD)] | p value |
|---|---|---|---|
| Age (years) | 17.25 (7.91) | 18.00 (3.65) | 0.510 |
| Height (cm) | 162.08 (12.48) | 167.02 (8.04) | 0.230 |
| Weight (kg) | 57.74 (15.85) | 65.68 (13.22) | 0.590 |
Ultrasound tendon thickness measures
When comparing the mean tendon thickness between dominant and non-dominant legs there was no significant difference in either group. Therefore each tendon was considered separately for analysis, making a total of 40 tendons in the study group and 34 in the control group.
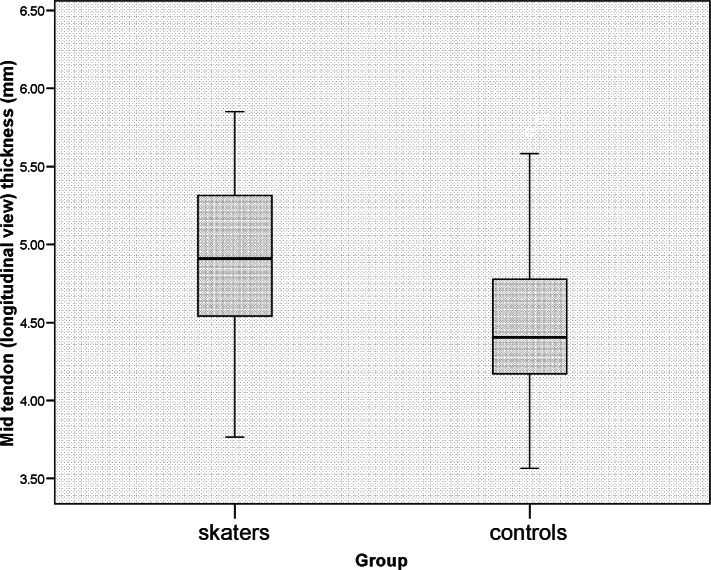
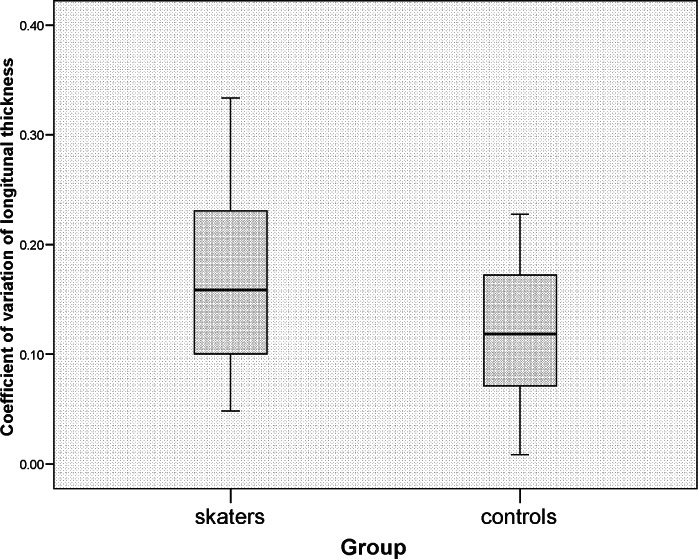
The mid-tendon thickness was significantly greater (p=0.001) in skaters [median (interquartile range) 4.91mm (4.54–5.31)] than in the control group [4.41mm (4.17–4.78)] (Fig. 2). The thickness at the MTJ was similar for both groups [skaters 4.77 (4.29–5.41), controls 4.84 (4.51–5.18), p=0.841], as was the calcaneal insertion thickness [skaters 3.90 (3.38–4.42), controls 4.07 (3.70–4.65), p=0.315] and the mean of the three thickness measures [skaters 4.63 (4.28–4.95), controls 4.45 (4.20–4.77), p=0.162]. The CoV of the three individual tendon measurements was significantly greater in the tendons of the skater group than those of the control group (p=0.017) (Fig. 3).
Figure 2.
Tendon thickness at the mid-point measurement in each group.
Figure 3.
COV of longitudinal tendon thickness in each group.
Ultrasound tendon thickness measure covariates
Age, height and weight did not differ between groups, so even if they had an association with tendon thickness this would not confound results. However, as training load differed between groups, its relationship with tendon thickness was regarded as important. In skaters, there was evidence of a relationship between present training load and mid tendon thickness (rho=0.45, p=0.03), longitudinal MTJ thickness (rho=0.35, p=0.03) and mean thickness (rho=0.39, p=0.01). There was no evidence of statistically significant correlations between training load and any thickness measures in controls.
Directly observed ultrasound measures of tendinopathy
Apart from measured tendon thickenings, no US markers of tendinopathy were seen in any symptomatic or asymptomatic skaters or controls.
Symptom status
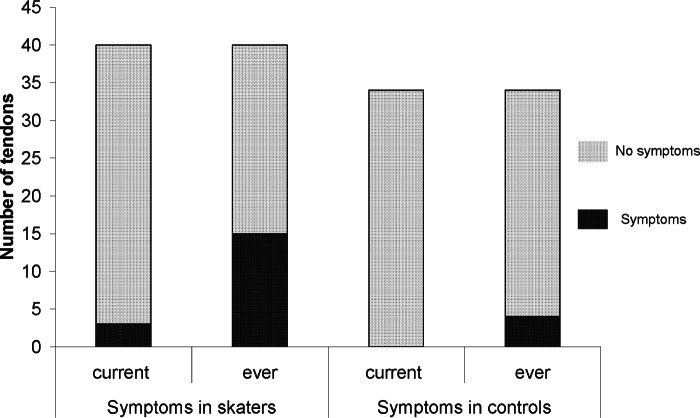
There was no significant difference between the groups when comparing current symptoms (Pearson Chi-Square=2.66, p=0.103). However, the figure skaters had experienced significantly more lifetime symptoms of Achilles tendinopathy (Pearson Chi-Square=6.38, p=0.012) (Fig. 4). None of the participants had ever ruptured an Achilles tendon.
Figure 4.
Number of Achilles tendons that had or had not been symptomatic.
For skaters, those with ‘symptoms ever’ had a median (IQR) calcaneal insertion thickness of 4.37mm (3.90–4.71), compared to 3.7mm (3.33–4.35) in those with no symptoms ever (p=0.01). There were no significant differences in US tendon thickness variables between ‘symptomatic ever’ and ‘asymptomatic ever’ groups in other thickness measures in skaters, or in any thickness measures in controls.
Similarly, skaters with present symptoms had a median (IQR) calcaneal insertion thickness of 4.71mm (4.57–5.02), compared to 3.9mm (3.36–4.37) in those with no present symptoms (p=0.030). There were no significant differences in US tendon thickness variables between presently symptomatic and presently asymptomatic groups in other thickness measures in skaters, or in any thickness measures in controls.
Reliability data
Intraobserver reliability for measurement of the tendon thickness measures was good to excellent19 with an ICC for the insertion of 0.89, an ICC of 0.88 for the myotendinous junction and an ICC for the mid-tendon of 0.96. Limits of agreement were 1.03mm, 0.96mm and 0.61mm respectively.
Discussion
Statement of principal findings
To our knowledge, this is the only study to show that figure skaters experience significantly more lifetime symptoms of Achilles pain than controls, which partially supports our primary hypothesis. However we were unable to demonstrate group differences in current symptoms.
The middle portion of the Achilles tendons of figure skaters was significantly thicker than those of controls, although there were no analogous findings for the other two thickness measures. We were unable to directly detect any signs of hypoechoic areas, neovascularisation, paratenon blurring or focal thickening in either group, thus failing to fully support our primary hypothesis. Nevertheless, we did calculate a greater CoV of longitudinal thickness within individual tendons of figure skaters. However the differences in measurements between the skaters and controls were small and all measurements were within a normal physiological range. This may support the increases in the skating group being due to a training adaptation, particularly as there was a significantly positive correlation between training volume and mid tendon thickness.
Our secondary hypothesis of the existence of asymptomatic US signs in skaters was unsupported, as no asymptomatic skaters had directly observable US abnormalities.
Strengths and weaknesses of the study
The main strength of this study was the representativeness of the sample of skaters. The sample covered all grades of ability and experience, included both male and female skaters, and encompassed most of the forms of the sport. Furthermore, the sample size was adequately powered to allow detection of group differences in Achilles mid-tendon thickness. Another important advantage was that the groups were well-matched in terms of the important confounders of gender, age, height and weight. Where the sample may have been limited was in the volume of training undertaken by skaters on a weekly basis. With a mean (sd) of 11.5 (6.0) hours a week, this is well below what elite skaters are likely to do and this may account for the lack of significant results and the small differences were significant results were found. In addition, bias was reduced by the investigator conducting the ultrasound scans being blinded to the symptomatic status of the subjects. This investigator received intensive sonographic training by a consultant musculoskeletal radiologist, and used established techniques and equipment. Consequently, reliability of measures was generally excellent. Another strength of this study is that it provides the only known source of data on Achilles tendon symptoms and signs in skaters.
This study had two potential limitations. The major confounder of training load, quantified by duration, was not matched between groups, and so any differences observed between groups in symptoms or pathological changes may have been due to the different training loads and not due to skating per se. Nevertheless, high training durations may be intrinsic to skating because of the time required for technical as well as physiological training, and so this criticism may not be valid. It is also important to note that training load did not differ between skaters with and without symptoms, suggesting that training load may not have contributed to differences in pathology between the groups. The second limitation was that although the study was powered to detect thickness differences, it was probably underpowered to detect differences in current symptoms, or to detect differences in all measures of thickness between skaters with and without symptoms.
Strengths and weaknesses in relation to other studies
This is the only study to our knowledge to investigate the relationship between ice skaters and the symptoms and sonographic signs of Achilles tendinopathy, and so it is not possible to compare this study with directly related papers. However it is very similar in approach to related Achilles tendinopathy prevalence studies on gymnasts18, and club track athletes20. Those studies18, 20 were similarly powered, were also matched for demographic variables, involved blinding of the investigator performing the scans to symptomatic status, and used the same sonographic techniques and equipment. Differing findings reflect the different sports surveyed. The lifetime prevalence of 37.5% in skaters compares closely to 35% in gymnasts18 and 42% in club runners20. These lifetime prevalences reflect the similar sporting demands of skating and gymnastics.
Meaning of the study: possible mechanisms and implications for clinicians or policymakers
Our finding of a more prevalent lifetime history of Achilles tendinopathy in the skaters suggests that skating training or competition are likely risk factors, and that those providing medical or rehabilitative care for skaters should be aware of the likelihood of this disorder. This may enable quicker symptom recognition and thus earlier treatment initiation, which may lead to a better outcome21. The lack of group differences for present symptoms may possibly be a type II error given the very small number of subjects with present symptoms, and further studies with larger numbers are required.
The greater mid-portion thickness in the skaters could be related to pathology, as previous work in non-skaters has shown greater tendon thickness with Achilles tendinopathy8,9. This link between thickness and pathology is supported by the fact that greater thickness was only noted in the mid portion of skater tendons, which is the most commonly injured part of the tendon6 .The fact that longitudinal midtendon thickness did not differ between skater tendons with or without symptoms weakens this supposed link between thickness and pathology, although it should be noted that this study was not powered to detect differences in tendon thickness within the skater group. It is possible that at least part of the greater mid-tendon thickness in the skaters could be a benign adaptive response to training22. This possibility is supported by the significant and positive correlation observed between training load and mid-tendon thickness and also by there being no pathological tendon changes in those with previous or current symptoms.
A particularly interesting finding was the association between greater calcaneal thickness and the existence of both past and present symptoms within the skater group. This suggests that greater calcaneal thickness in skaters may be associated with pathology, though exactly what pathology is not possible to tell from this study; i.e. reported pain currently or previously in the Achilles tendon may represent mid portion Achilles tendinopathy, paratenonitis, insertional tendinopathy or other diagnosis. However, there were no differences between skaters and non-skaters in calcaneal thickness, even though these groups differed in symptom-atology. One way to explain this apparent paradox is to surmise that healthy skaters may tend towards smaller calcaneal insertion thicknesses than the age and height-matched general population, but that these may thicken in response to pathology. This is supported by post hoc comparison of the calcaneal thickness in currently asymptomatic skaters and asymptomatic non skaters which did indeed suggest a trend for a smaller calcaneal insertion thickness in healthy skaters, although this was not significant (p=0.15). Alternatively this may represent of the study not being powered for this comparison. The lifetime prevalence of Achilles disorders in figure skaters is lower than many other overuse injuries; 10 out of 469 overuse injuries reported in one retrospective study of junior elite figure skaters, albeit a group less likely to develop tendinopathy due to age but comparable tour study group.
Although we did not detect any standard sonographic markers of tendon abnormality in either group, we did calculate a greater co-efficient of variation (CoV) of longitudinal thickness within individual tendons of figure skaters, initially suggesting that they may be more prone than the general population to areas of focal thickening, which are recognised indicators of pathology23. However, it has been shown that the patella tendon may adapt to load by increasing the variation of thickness along its length24, indicating that our results may largely reflect an adaptive, rather than pathological, process in the skaters25. This lack of a relation to pathology was supported by a post-hoc analysis showing a surprising trend (P=0.056) for the currently asymptomatic skater tendons to have larger thickness variations than the 3 currently symptomatic skater tendons (CoV of 18% compared to 10%).
The lack of any directly observed markers of degeneration in all subjects is surprising, but probably reflects the fact that only 3 tendons were currently symptomatic. Other studies have also reported a lack of US signs in some symptomatic tendons3, 8 which does suggest that US signs may be a less sensitive indicator of tendinopathy than assumed.
Unanswered questions and future research
The most important unanswered question is whether sonographic abnormalities exist in asymptomatic skaters. If further work in samples powered to detect such an effect shows that they do, further longitudinal work may be carried out to assess if such asymptomatic signs are actually pre-symptomatic signs; that is, markers for progression to symptomatic pathology. Subsequent work could involve the testing of prophylactic treatments in skaters.
Another issue revealed by this study is the role of the calcaneal insertion region in skater pathology. This study showed that only calcaneal insertion thickness had an association with past and present pathology in skaters, and this finding deserves further investigation. For example, an intriguing question might be to confirm whether healthy skaters have smaller calcaneal insertion thicknesses than the normal population, and whether this difference has some role in the development of Achilles tendinopathy in skaters.
Finally, further studies should be adequately powered for sub-group analysis, and detecting sufficient participants with current symptoms. This will allow fuller evaluation of mechanisms, and a greater opportunity to examine the relationship between skating, current symptoms and sonographic abnormalities.
Acknowledgments
We would like to thank Dr. Claire Emerson and Dr. Sanjay Vijayanathan for their help and advice during this study. Thanks also to Dawn Peckett, Sally Hayter and King Edward VI School, Southampton for their help with recruitment. No funding was received for this work.
APPENDIX 1
Questionnaire
Section 1:
1. Code number:.......................
2. Date of Birth:.......................
3. Gender: Male/Female
4. Figure Skating discipline: Singles/Pairs/Dance/Synchronised
5. Level of competition: Novice/Junior/Senior
Section 2:
6. Which is your dominant leg? (Take off leg): Right/Left
7. How many hours of training do you do per week? On ice:....../Off ice:......./Total:.........
8. How many years have you been training at this intensity?.................
Section 3:
9. Have you ever had pain, tenderness or stiffness in your Achilles tendon? Right: Yes/No: Left: Yes/No.
If the answer to question 9 is no for both legs, you have finished the questionnaire.
If the answer is yes for either leg, please continue to question 10.
10. Is your Achilles tendon painful, tender or stiff today? Right: Yes/No: Left: Yes/No.
11. Has your Achilles tendon been painful, tender or stiff at any time during the last month? Right: Yes/No: Left: Yes/No.
12. Has your Achilles pain, tenderness or stiffness ever lasted for more than 3 months continuously? (i.e. it hurt more or less every day)? Right: Yes/No: Left: Yes/No
13. Has your Achilles pain, tenderness or stiffness ever lasted for more than 3 months, off and on (i.e. it hurt at least once a week but not every day)? Right: Yes/No: Left: Yes/No
14. What age did you first get Achilles tendon pain?....................
15. Have you ever sought health professional advice/treatment? Yes/No
16. What investigations have you had?...........................
17. What treatment have you received (surgery, plastercast, physio, other)?.........................
18. How much training did you miss (days, weeks, months or years)?..............................
19. Was your Achilles pain initially caused by a specific injury or incident? Yes/No
20. Have you ever ruptured your Achilles tendon? )? Right: Yes/No: Left: Yes/No
21. If yes, when did you rupture your Achilles tendon? Right: (dd/mm/yyyy): left (dd/mm/yyyy)
22. What treatment did you receive? (surgery, plastercast, physio, other):...................
23. How much training did you miss? (days, weeks, months or years):......................
References
- 1.Smith AD, Ludington R. Injuries in elite pair skaters and ice dancers. Am J Sports Med. 1989;17:482–488. doi: 10.1177/036354658901700406. [DOI] [PubMed] [Google Scholar]
- 2.Fortin J. Competitive figure skating injuries. Pain Physician. 2003;6:313–318. [PubMed] [Google Scholar]
- 3.Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. Sports Med. 1995;20:77–89. doi: 10.2165/00007256-199520020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffulli N, Khan K, Puddu G. Overuse Tendon Conditions: Time to Change a Confusing Terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 6.Gibbon W, Cooper J, Radcliffe G. Distribution of sonographically detected tendon abnormalities in patients with a clinical diagnosis of chronic achilles tendinosis. J Clin Ultrasound. 2000;28:61. doi: 10.1002/(sici)1097-0096(200002)28:2<61::aid-jcu1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Khan K, Forster B, Robinson J, Cheong Y, Louis L, Maclean L, Taunton JE. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Brit J Sports Med. 2003;37:149–153. doi: 10.1136/bjsm.37.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehrer S, Breitenseher M, Brodner W, Kainberger F, Fellinger EJ, Engel A, Imhof F. Clinical and sonographic evaluation of the risk of rupture in the Achilles tendon. Arch Orthop Trauma Surg. 1997;116:14–18. doi: 10.1007/BF00434093. [DOI] [PubMed] [Google Scholar]
- 9.Kainberger F, Mittermaier F, Seidl G, Parth E, Weinstabl R. Imaging of tendons - adaptation, degeneration, rupture. Eur J Radiol. 1997;25:209–222. doi: 10.1016/s0720-048x(97)00058-2. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21:158–162. doi: 10.1136/bjsm.21.4.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 12.Nichol A, McCurdie I, Etherington J. Use of ultrasound to identify chronic Achilles tendinosis in an active asymptomatic population. J R Army Med Corps. 2006;152:212–216. doi: 10.1136/jramc-152-04-03. [DOI] [PubMed] [Google Scholar]
- 13.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 14.Fredberg U, Bolvig A. Significance of Ultrasonographically Detected Asymptomatic Tendinosis in the Patellar and Achilles Tendons of Elite Soccer Players. A Longitudinal Study. Am J Sports Med. 2002;30:488–491. doi: 10.1177/03635465020300040701. [DOI] [PubMed] [Google Scholar]
- 15.Cook J, Khan K. The treatment of resistant, painful tendinopathies results in frustration for athletes and health professionals alike. Am J Sports Med. 2003;31:327–328. [PubMed] [Google Scholar]
- 16.Shrout PE, Fleiss JL. Intraclass correlation: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman GA. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8476:307–330. [PubMed] [Google Scholar]
- 18.Emerson C, Morrissey D, Perry M, Jalan R. Ultrasonographically detected changes in Achilles tendons in elite gymnasts in the UK compared with controls – an observational study. Man Ther. 2010;15:37–42. doi: 10.1016/j.math.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Portney LG, Watkins MP. Foundations of Clinical Research - application to practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2000. p. 565. [Google Scholar]
- 20.Shaikh Z, Perry M, Morrissey D, Ahmad M, Buono A, Maffulli N. Achilles Tendinopathy in Club Runners. Int J Sports Med. 2012 Feb 29; doi: 10.1055/s-0031-1299701. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Alfredson H. In: Pain in the Achilles Region. Chapter 28 in: Clinical Sports Medicine. 3rd Edition. Bruckner P, Khan K, editors. McGraw-Hill Publishing; New York: 2005. [Google Scholar]
- 22.Kongsgaard M, Aagaard K, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol. 2005;99:1965–1971. doi: 10.1152/japplphysiol.00384.2005. [DOI] [PubMed] [Google Scholar]
- 23.Yu JS, Popp JE, Kaeding CC, Lucas J. Correlation of MR imaging and pathologic findings in athletes undergoing surgery for chronic patellar tendiniti. Am J Roentgenol. 1995;165:115–118. doi: 10.2214/ajr.165.1.7785569. [DOI] [PubMed] [Google Scholar]
- 24.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. 2007;191:111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 25.Dubravcic-Simunjak S, Pecina M, Kuipers H, Moran J, Haspl M. The incidence of injuries in elite junior figure skaters. Am Journal Sports Med. 2003;31(4):511–517. doi: 10.1177/03635465030310040601. [DOI] [PubMed] [Google Scholar]