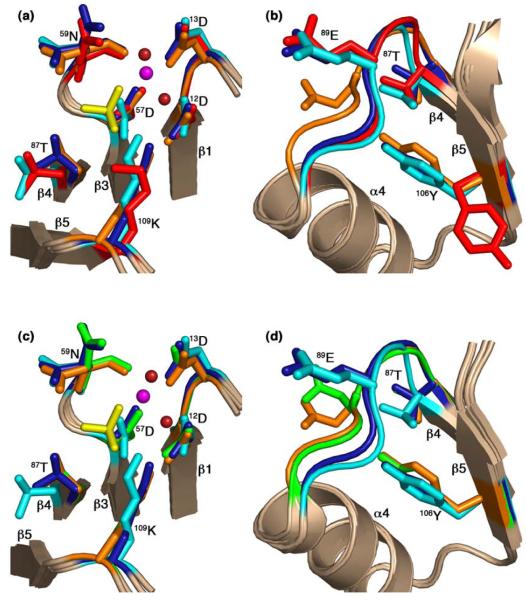
Figure 6.
Comparison of active-site and switch residues in the CheY-CheZ200-214 complexes with those in inactive and active CheY. CheY molecules in BeF3−-free F432YZ200-214 (key features shown in cyan), BeF3−-bound F432YZ200-214 (key features shown in deep blue) and BeF3−-bound P2(1)2(1)2YZ200-214 (key features shown in orange) are superposed on inactive Mg2+-bound CheY (2CHE)14 (key features shown in red) in (a) and (b) and on BeF3−-activated CheY (1FQW)15 (key features shown in green) in (c) and (d), focusing on the active site in (a) and (c), and on the activation-sensitive α4β4α5 region in (b) and (d). The side chains of key active-site and switch residues are illustrated as ball and stick models. The Mg2+ ions (magenta), coordinating water molecules (deep red) and the BeF3−-complexes (yellow) at the active site in BeF3−-bound F432YZ200-214 are shown in (a) and those in BeF3−-bound P2(1)2(1)2YZ200-214 are shown in (c). The BeF3−-free F432YZ200-214 and the “metaactive” conformer of the BeF3−-bound F432YZ200-214 structures solved from crystals grown in Tris (pH 8.4) are used as representatives of F432YZ200-214 structures in this figure.