Table 1.
ERα and ERβ relative binding affinity (RBAs) of compounds 2a–m, 4a–f, 6a–b and 8a–b.a
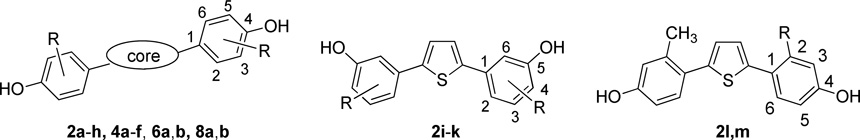 | ||||||
|---|---|---|---|---|---|---|
| 2a–h, 4a–f, 6a,b, 8a,b | 2i–k | 2l,m | ||||
| entry | cmpd | core | R | ERαb | ERβb | β/α |
| 1 | 2a | H | 0.040 ± 0.004 | 0.79 ± 0.11 | 19.8 | |
| 2 | 2b | 2-Me | 1.43 ± 0.19 | 1.74 ± 0.26 | 1.2 | |
| 3 | 2c | 3-Me | 0.004 ± 0.001 | 0.002 | 0.5 | |
| 4 | 2d | 3,5-diMe | 0.003 ± 0.001 | 0.005 ± 0.001 | 1.7 | |
| 5 | 2e | 2-F | 2.03 ± 0.19 | 33.1 ± 5.8 | 16 | |
| 6 | 2f | 2-Cl | 6.7 ± 1.3 | 10.0 ± 2.4 | 1.5 | |
| 7 | 2g | 3-F | 0.013 ± 0.004 | 0.142 ± 0.004 | 11 | |
| 8 | 2h | 3-Cl | 0.009 ± 0.001 | 0.036 | 4.0 | |
| 9 | 2i | 2-F | 0.008 ± 0.002 | 0.066 ± 0.066 | 8.3 | |
| 10 | 2j | 3-F | 0.009 ± 0.006 | 0.041 ± 0.004 | 0.4 | |
| 11 | 2k | 4-F | ~0.001 | 0.010 ± 0.002 | 10 | |
| 12 | 2l | F | 0.97 ± 0.01 | 0.91 ± 0.26 | 0.94 | |
| 13 | 2m | Cl | 4.29 ± 0.64 | 5.89 ± 0.21 | 1.4 | |
| 14 | 4a | 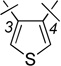 |
H | 0.57 ± 0.12 | 1.69 ± 0.45 | 2.9 |
| 15 | 4b | 2-Me | 2.16 ± 0.54 | 4.9 ± 1.3 | 2.25 | |
| 16 | 4c | 3-Me | 0.830 | 0.297 | 0.36 | |
| 17 | 4d | 3,5-diMe | 0.039 ± 0.003 | 0.004 ± 0.001 | 0.10 | |
| 18 | 4e | 2-F | 3.03 ± 0.85 | 9.1 ± 2.2 | 3.0 | |
| 19 | 4f | 2-Cl | 13.07 ± 1.1 | 17.5 ± 2.1 | 1.3 | |
| 20 | 6a | 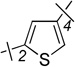 |
2-F | 1.22 ± 0.32 | 10.4 ± 1.2 | 8.5 |
| 21 | 6b | 2-Cl | 4.21 ± 0.54 | 25.6 ± 5.7 | 6.1 | |
| 22 | 8a | 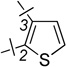 |
2-F | 1.22 ± 0.35 | 2.54 ± 0.76 | 2.1 |
| 23 | 8b | 2-Cl | 6.50 ± 0.61 | 7.73 ± 2.1 | 1.2 | |
To simplify comparisons between compounds in closely related series, we designate locant positions of the substituents on the phenyl groups with respect to the thiophene core; locant positions within the thiophene core itself are given by numbers in italics.
Relative binding affinity (RBA) values are determined by competitive radiometric binding assays and are expressed as IC50estradiol/IC50compound×100 ± the range or standard deviation (RBA, estradiol = 100%). In these assays, the KD for estradiol is 0.2 nM on ERα and 0.5 nM on ERβ.51 For details, see the Experimental Section.
