Abstract
Oncolytic poxviruses have demonstrated initial promising results in patients with cancer in clinical trials, yet further improvements are needed. It has been shown that a single point mutation in the A34R gene resulted in the production of more total progeny virus and more extracellular enveloped virus (EEV), a form that can be immune-evasive and with enhanced spread. We have genetically engineered a new oncolytic poxvirus (designated vA34R) by incorporating this mutated A34R gene into a viral backbone (vvDD) which was designed for tumor-selective replication. This rationally designed virus can evade neutralization from antipoxvirus antibodies and is highly cytotoxic to cancer cells. It demonstrates improved spread and increased replication within the peritoneal cavity resulting in improved antitumor effects in a peritoneal carcinomatosis (PC) model of MC38 colon cancer. Impressively, after carrier cell-mediated delivery in the preimmunized host, vA34R displayed high replication in tumor nodules yet low accumulation in normal tissues thus enhancing the therapeutic index leading to 70% long-term cures. These results demonstrate that vA34R gains an enhanced therapeutic index for PC via immune evasion, increased spread, and production of more progeny virus. Thus, vA34R may be a potent oncolytic virus (OV) for patients with PC, even after prior exposure to vaccinia virus (VV).
Introduction
The use of oncolytic viruses (OVs) as targeted cancer therapy has demonstrated significant promise in clinical trials.1,2,3 Vaccinia virus (VV), most well known for its role in the eradication of smallpox, has been an attractive vector for vaccines, oncolytic virotherapy, and gene therapy over the last 30 years. This has been attributed to its unique properties, especially for its native tumor tropism, efficient cell–cell spread and high levels of transgene expression in tumor cells.4,5,6 A number of investigators have engineered multiple oncolytic VV and demonstrated their efficacy and safety in preclinical studies.7,8,9,10,11 So far, phase I and II trials of various oncolytic VV have shown clinical responses and minimal therapy-associated toxicities in patients.12,13 However, more improvements will be needed in order for the oncolytic VV to be highly efficacious and safe in patients with cancer.
Analysis of VV genomics and cancer biology provides some valuable insights to potential strategies for further development of oncolytic VV. Wild-type VV manages to infect cells derived from a broad range of histologies. Multiple genes expressed by the virus can be deleted to create tumor-selective replication, including genes required to synthesize nucleotide resources,14 and the vaccinia growth factor (vgf) gene, which produces a protein homologous to epidermal growth factor secreted by VV-infected cells, thus stimulating surrounding noninfected cells via the EGFR-Ras pathway to divide and prime them for vaccinia replication.15 As abundant cytoplasmic resources are already available in rapidly dividing cancer cells, the deletion of these genes (tk and vgf), individually and together, has demonstrated enhanced tumor selectivity and hence increased safety of the virus.7,14
Productive replication of poxviruses results in the formation of fully functional virions called intracellular mature virions (IMV), some of which are then transported to the exterior of the cell after the incorporation of an extra-host cell derived membrane.5 The latter form of virion, called the cell-associated enveloped virion, stimulates polymerization of cytoplasmic actin tails beneath them, whereas the remaining attached to the host cell by means of A34R, a virus-encoded type II integral membrane glycoprotein.16,17 The actin tails project the cell-associated enveloped virion into adjacent cells resulting in efficient cell–cell spread, eventually leading to a death zone expanding centrifugally from the site of infection. Rarely, the cell-associated enveloped virion gets completely detached from the host cell to form extracellular enveloped virions (EEV).18
VV gene A34R plays important roles in the release of cell-associated enveloped virion to become EEV and in the infectivity of EEV virions. It encodes an EEV-specific glycoprotein with homology to C-type lectins. The A34R protein affects plaque formation, EEV release, EEV infectivity, and virus virulence.16,19 Interestingly, the IHD-J strain of VV naturally produces up to 40 times more EEV (nearly 30% of the progeny) than does the related WR strain with no loss of infectivity.16 The open reading frame of the A34R gene of the IHD-J strain differs from that of the WR strain by six nucleotides resulting in two amino-acid changes, only one of which (lysine-151 → glu) was found to be responsible for enhanced EEV production.16 Some important, yet overlooked, properties include faster entry of target cells by the EEV form of the virus,20 and higher total yields of infectious virus.16,19
The EEV form has several unique advantages in that they are (i) released early during the infection cycle,21,22 enabling faster spread; (ii) enhanced entry into target cells without induction of cell signaling, unlike IMV;23,24 (iii) capable of long-range dissemination inside the host;5,21,25 (iv) resistant to complement neutralization;22,26 and (v) resistant to VV-specific antibody neutralization due to the stealth provided by the outer membrane.22,27 Despite these advantages, there are two major hurdles for the exploitation of EEV in the therapeutic setting. First is that the outer membrane of EEV is extremely fragile, being easily ruptured by low pH, freeze-thawing or polyanions, making it almost impossible to isolate considerable quantities of EEV for any meaningful usage.27,28 Second, the amount of EEV produced by most strains of wild-type VV are very low, <1% of all infectious viral progeny.21,25 In a proof of principle study, one of the authors of this study (S.H.T.) showed that such a genetically engineered VV with insertion of the IHD-J variant of the A34R gene into the WR strain of VV lacking its own A34R gene was efficient in producing EEV virions capable of spreading from subcutaneous tumors to lung metastases.10 However, other therapeutic advantages associated with the EEV-highly producing OV were not explored in the previous study.10
Oncolytic virotherapy has been investigated as a novel approach to the treatment of peritoneal carcinomatosis (PC).29,30,31,32,33 PC is a common lethal sequelae of several abdominal cancers. PC is a terminal metastatic condition characterized by the presence of numerous tumor nodules on the peritoneal surface due to the free spread of tumor inside the peritoneal cavity. It may be primary, arising de novo from the peritoneal mesothelial cells, or more commonly secondary to primary tumors arising from the gastrointestinal tract or ovaries. Once diagnosed, the prognosis is dismal. The benefit of systemic chemotherapy has been minimal due to the poor vascularity of these nodules.34,35 However, radical surgery in the form of tumor debulking to remove all macroscopic tumors, followed by intraperitoneal chemotherapy to target remaining microscopic remnants, has been associated with improved survival,36 thereby proving in principle that a regional approach which focuses on reduction of tumor burden in the peritoneal cavity can prolong survival. Unfortunately, only a small proportion of patients with PC are candidates for such radical surgery and the resultant operative morbidity and mortality are significant enough to warrant a search for an alternative or complementary treatment modality.37,38
In this study, we constructed and studied an A34R specifically mutated oncolytic VV with tumor-selective mutations in the vgf and tk genes. We hypothesize that a new virus with combined properties of tumor-selective replication, and potent immune evasion and virus spreading (A34R mutation), would function well in tumor models such as PC. We also hypothesized that carrier cell-based delivery of this oncolytic agent into the peritoneal cavity (direct intraperitoneal delivery of syngeneic cancer cells loaded ex vivo with vA34R virus in IMV form), could allow for the initial immune evasion for IMVs until further stealth transmission is taken over by EEVs produced in vivo, thereby evading humoral anti-VV immunity. Our results demonstrate that the new A34R mutant VV is an immune-evasive OV with enhanced therapeutic efficacy in the PC model derived from murine colorectal cancer.
Results
Construction and initial characterization of the new vA34R and vvDDr
We constructed two new viruses by transfecting two new shuttle plasmids (for A34R locus), pA34Rm-DsRed or pA34Rwt-DsRed, respectively into CV-1 cells and then infected the cells with vvDD-CD at an multiplicity of infection (MOI) of 0.1, as described in Materials and Methods. The new recombinant viruses, vA34R that contains the single mutation for K151E in the A34R gene and its revertant vvDDr that contains no mutation in the A34R gene, were isolated by screening for DsRed (red fluorescent protein) expression and plaque purification (Supplementary Figure S1). The viruses were confirmed by DNA sequencing and formation of the comet plaque phenotype by vA34R.
vA34R virus produced more EEV and total progeny virus, and exerted higher cytotoxicity in cancer cells
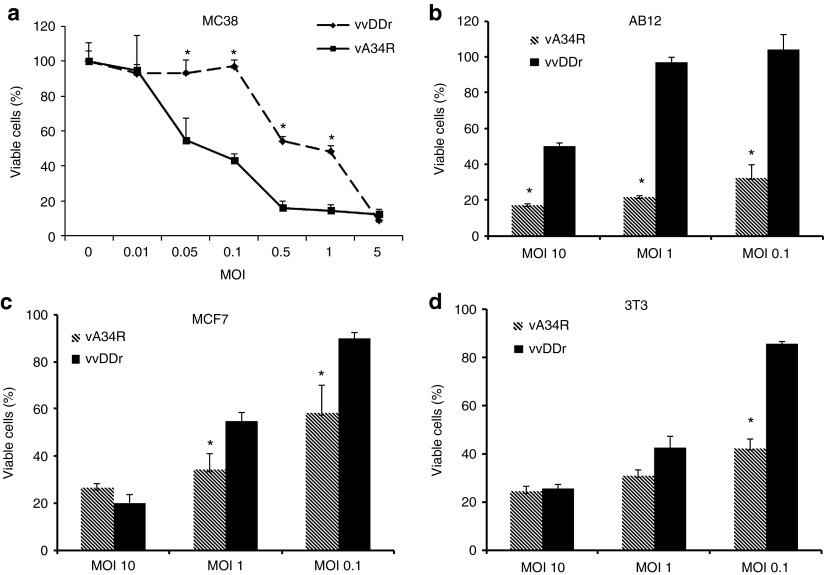
We tested the cytotoxic potential of vA34R and vvDDr against MC38 tumor cells and other cancer or immortalized cells using a standard cytotoxicity (MTS) assay (Figure 1a). In MC38 colon cancer cells at 48 hours after infection, vA34R displayed much better cytotoxicity when used at multiple MOIs between 0.05 and 1.0. However, at high viral dosage (MOI = 5 or greater), essentially all MC38 cancer cells were dead and both viruses displayed potent cytotoxicity (Figure 1a). This pattern was essentially reproduced in AB12 mesothelioma cells (Figure 1b), MCF7 breast cancer cells (Figure 1c), and immortalized NIH3T3 (Figure 1d) cells. The only exception was AB12 cells where the different cytotoxicity was still observed at an MOI of 10. This may be due to the relatively poor infectivity of AB12 cancer cells by VV (data not shown). Although NIH3T3 are not cancer cells, they are readily killed by VV as they are immortalized and dividing.
Figure 1.
Cytotoxicity in vitro. (a) Viral cytotoxicity (measured by MTS assay) of mouse colon cancer (MC38) cells at 48 hours at multiple MOIs showing the percentage of viable cells after both vA34R and vvDDr treatment; vA34R is more cytotoxic than vvDDr at MOIs of 0.05, 0.1, 0.5, and 1; at MOI of >1, there is no difference as almost all cells gets killed at higher MOIs by 48 hours. Cytotoxicity at MOIs of 0.1, 1, and 10 for (b) AB12, (c) MCF7, and (d) 3T3 cells at 72 hours are presented. *P < 0.05. MOI, multiplicity of infection.
We next analyzed the production of EEV and total progeny viruses in MC38 cancer cells (Figure 2). 1.0 × 105 MC38 cells were seeded in six-well plates and the next day infected with either virus at MOIs of 0.1, 1.0, and 10. At 48 hours after infection, supernatant (for EEV virus) or supernatant plus all cells (for total progeny virus) were harvested, and infectious viruses were quantified by plaque assays in CV-1 cells (Figure 2). At different viral dosages (MOIs of 0.1, 1, and 10), the yields of total virus (both IMV and EEV) from vA34R were always higher than vvDDr (two to over tenfold), whereas the yields of EEV form produced from vA34R were 5- to 20-fold more than that from vvDDr. Our results are in agreement with early studies on A34R gene in VV.16,19 Therefore, vA34R virus, with the point mutation in the A34R gene, enhanced the production of both the EEV form and total progeny virions.
Figure 2.

Production of EEV form and total progeny viruses. MC38 cancer cells were infected at MOIs of 0.1, 1, and 10, their supernatant or supernatant and cells were collected at 48 hours. Virus titers from the supernatants alone and from both supernatants and cells (total) of both vvDDr and vA34R-infected MC38 cells at (a) MOI 0.1, (b) MOI 1, and (c) MOI 10 is presented. vA34R is recovered at higher titers from both the supernatants and cells plus supernatant (total). MOI, multiplicity of infection; PFU, plaque-forming unit.
Ability for remote spread in vitro
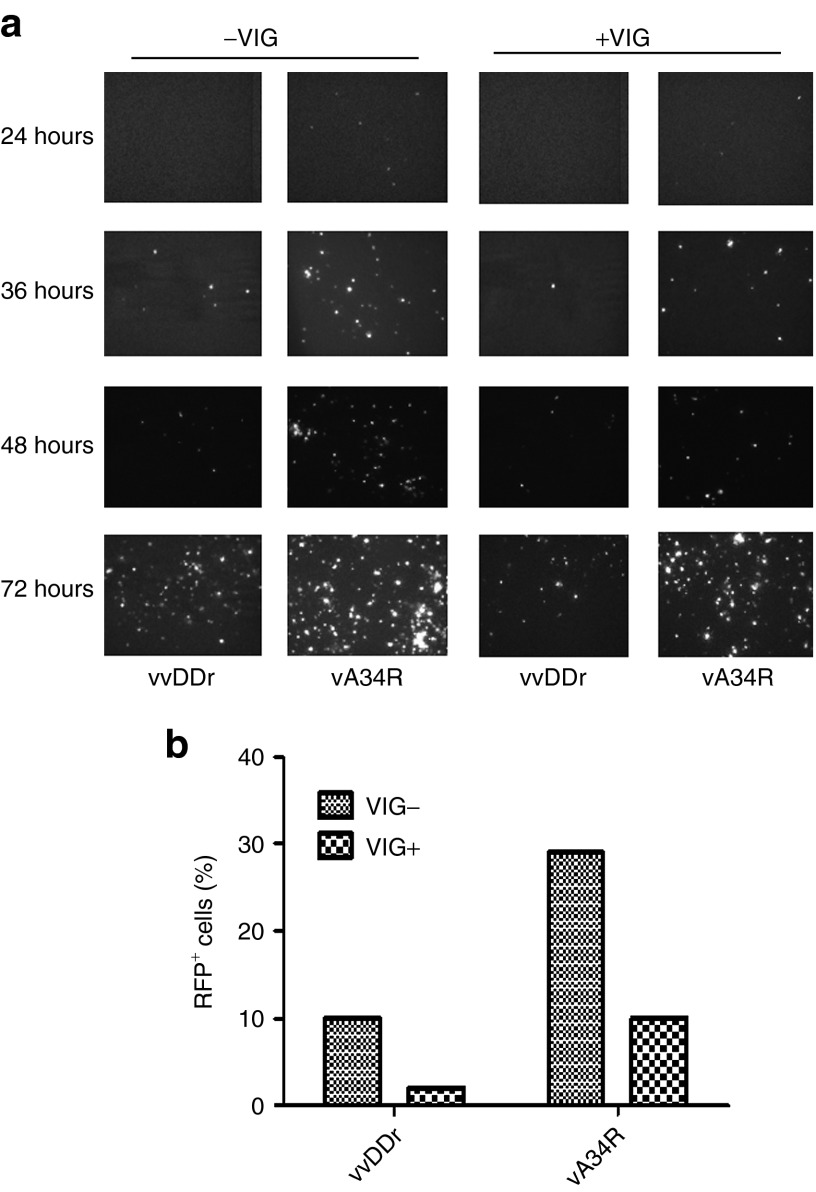
To test whether vA34R virions are shed into the supernatant and mediate effective spread to remote tumor sites, we decided to simulate the PC model in vitro by using a transwell setup. Both the bottom and insert wells were seeded with MC38 cancer cells mimicking two distant tumor nodules in PC. The bottom wells were infected with either vvDDr or vA34R at an MOI of 1.0, and the top uninfected insert wells were observed for red fluorescent protein (DsRed) expression at 24, 36, 48, and 72 hours. The DsRed expression in the originally uninfected insert wells demonstrates the ability of EEV forms of virus released in the supernatant from the bottom infected wells to spread to distant sites. Fluorescence microscopic pictures taken at several time points indicated an increased expression of DsRed positive cells in the insert well for vA34R when compared with vvDDr, both in the presence and absence of human antivaccinia immunoglobulin (VIG) (Figure 3a). When VIG was used in an effort to mimic host antiviral immunity, the infectivity of remote MC38 cells was reduced for both viruses, yet a similar pattern of vA34R displaying higher remote spreading was observed. At 72 hours, all cancer cells in the insert wells were harvested and pooled samples of the triplicates in each category were flow sorted to quantify the number of DsRED-expressing virally infected cells when compared with uninfected cells. In the presence of VIG, the amount of viral DsRED expression was decreased overall as expected; however, vA34R was more effective than vvDDr in infecting the remote cells by eightfold (Figure 3b). Overall, vA34R in the presence of VIG was as effective as vvDDr in the absence of VIG for remote infection.
Figure 3.
Remote spread in vitro by transwell assay. (a) Florescence microscopy pictures showing the increased expression of DsRed from vA34R virally infected cells at 24, 36, 48, and 72 hours after infection from the insert wells in both the presence and absence of vaccinia immunoglobulin (VIG), when compared with vvDDr. (b) Flow sorting analysis measuring the DsRED fluorescence in infected cells compared with mock (uninfected) cells in the insert wells at 72 hours shows a threefold and fivefold increase in the percent of DsRed (RFP+) fluorescent gated cells with vA34R compared with vvDDr in the absence and presence of VIG, respectively. Due to pooling of all triplicate wells for flow sorting, no means or median values were available.
Viral infectivity
We further examined the infectivity of the two viruses in vitro before performing in vivo experiments. We have examined the infectivity of MC38 cancer cells and CV-1 cells at MOIs ranging from 0.01 to 25 (Supplementary Figures S2 and S3). First, we examined the production of infectious virus in MC38 cells at 12 hours after infection. In MC38 cancer cells infected at MOIs of 1, 5, and 10, vA34R always produced more infectious progeny virus than vvDDr (Supplementary Figure S2a). At MOI of 1.0, the yield of vA34R was 1.5E6 plaque-forming unit (pfu) (per 5.0E5 cells) verses vvDDr at 2.0E5 pfu. The difference was significant at this and higher MOIs. Then we examined the infectivity by analyzing the percentage of infected cells infected at 12 hours. Cells at 12 hours after infection were flow sorted into MC38 cells in 96-well plates and incubated for an additional 48 hours and analyzed for DsRED expression and CPE as indicators of original cells being infected. At an MOI of 0.01, only less than 1% of MC38 cancer cells were infected by either virus. The percentage of infected cells increased with higher MOIs. At an MOI of 1.0, the infectivity of vA34R increased to ~32% and vvDDr at ~14% in MC38 cells (Supplementary Figure S2b). Due to the intrinsic error of flow sorting, these results may underestimate the actual infectivity rate. These results together suggested that vA34R was able to infect more cells when examined at 12 hours after infection. It would not be expected for the IMV form of vA34R to infect better than the IMV form of vvDDr, and it is presumed that the majority of purified vaccinia to be in the IMV form (the EEV envelope is fragile and does not withstand the purification process). These results suggest that there may be more EEV in the preparation or more efficient infection and spread of vA34R virus, even at 12 hours after infection.
Ability for remote spreading in vivo
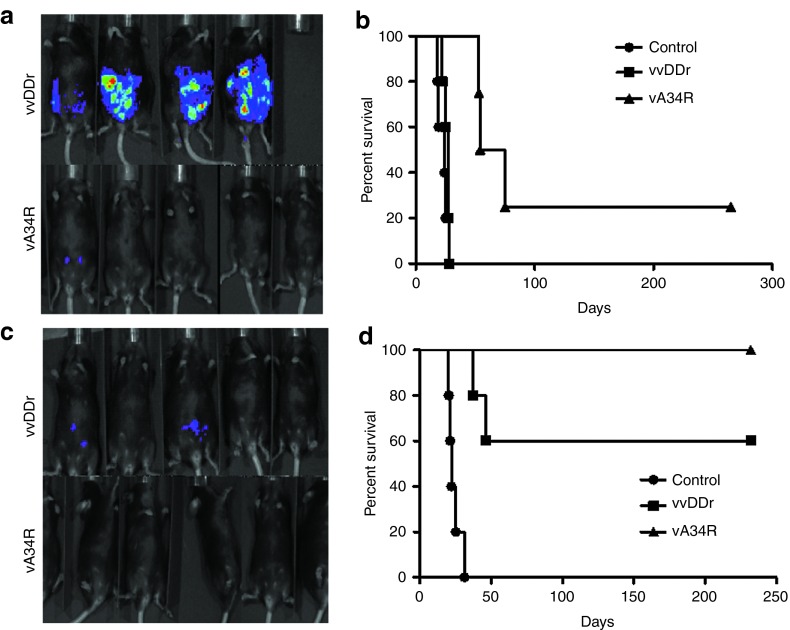
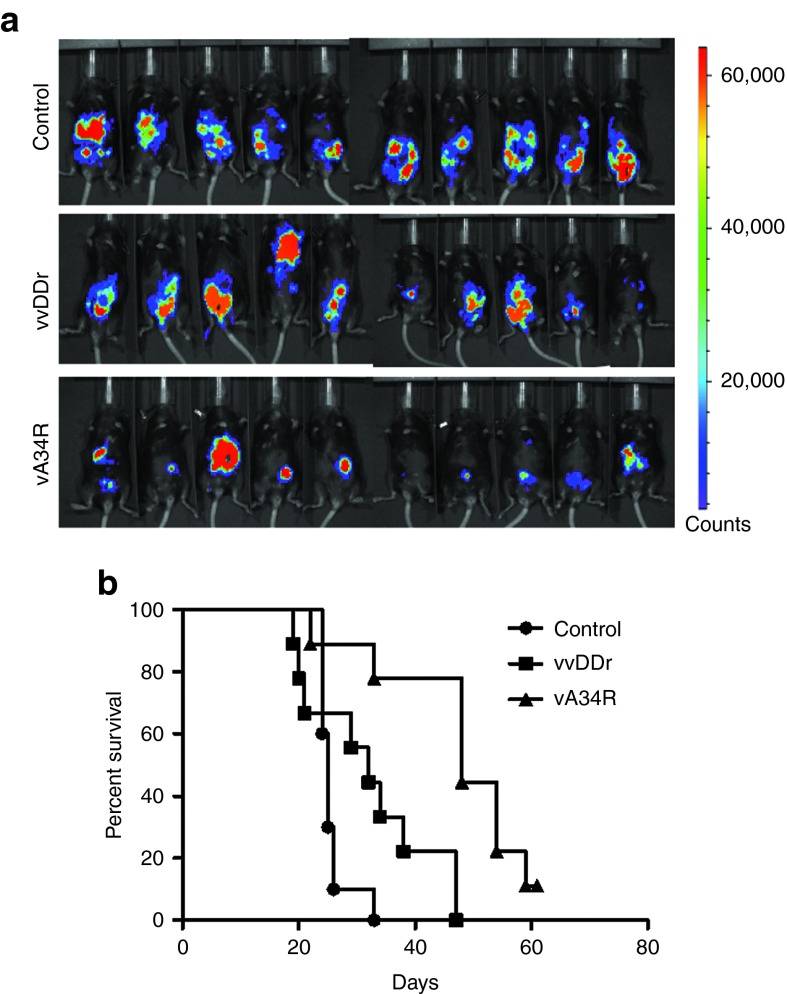
We then tested the ability for remote spread of the viruses in vivo, in tumors inside the peritoneal cavity. We injected a suspension of luciferase-tagged MC38 (MC38-luc) cells, which were either mock infected or infected with vvDDr or vA34R at a very low MOI (0.001) into naïve C57BL/6 (B6) mice i.p. At such a low MOI, less than 1% of cells were infected (Supplementary Figure S2). In vivo bioluminescence imaging for luciferase activity on day 7 after injection showed diffuse peritoneal tumor cells in all mice injected with tumor cells that were infected with vvDDr or mock infected. In contrast, there was no visualized tumor-associated luciferase activity seen in the vA34R cohort (Figure 4a), reflecting the ability of this virus to spread to noninfected cancer cells in vivo and eliminate them. On long-term follow-up for survival, all mice in the mock-infected and vvDDr-infected groups developed PC, with no statistical difference in survival. However, mice with vA34R-infected cancer cells survived longer and one out of four survived to the end of the experiment (~270 days) (Figure 4b).
Figure 4.
Remote spread in vivo. (a) In vivo bioluminescence imaging for the formation of MC38-luc tumor by uninfected cells when the same were infected with either vA34r or vvDDr at an MOI of 0.001 ex vivo. Tumor burden visualized by luciferase activity in naïve B6 mice, 7 days after i.p. injection of 1.0 × 106 MC38-luc cells infected ex vivo with either virus at an MOI of 0.001 or mock infected (control). (b) Kaplan–Meier analysis of these mice revealed that vA34R cohort developed PC slower and less often, thus resulting in prolonged survival compared with the vvDDr cohort, all of which developed PC (median survival, 54 versus 25 days, P = 0.002) and mock-infected mice (median survival, 54 versus 19 days, P = 0.00184). There was no statistical difference between the vvDDr and control cohorts. (c) In vivo bioluminescence imaging in preimmunized mice in both vvDDr and vA34R groups where the viruses are infected ex vivo in MC38-luc cells at an MOI of 0.01. (d) Kaplan–Meier analysis of these VV-immunized mice after i.p. injection of 1.0 × 106 MC38-luc cells infected ex vivo with vvDDr or vA34R at an MOI of 0.01 or mock infected (control). Median survival of vvDDr group and vA34R group (>232 days) were longer than the mock-infected group (25 days) in a statistically significant fashion (log rank test, P = 0.003). MOI, multiplicity of infection.
We then increased the viral dose to infect the cancer cells at an MOI of 0.01 (tenfold higher) and repeated the experiment (Figure 4c,d). We observed the tumor formation to be diminished further (Figure 4c), and thus the animal survival was longer for both groups treated with vA34R and vvDDr. In fact, mice incubated with MC38 cells infected with vA34R all survived to the end of the experiment (232 days). Median survival of the vvDDr group and the vA34R group (>232 days) were longer than the mock-infected group (25 days) in a statistically significant fashion (log rank test, P = 0.003).
In summary, these data suggest that both viruses can replicate and spread between tumor nodules in vivo to exert their antitumor effects, with the vA34R virus displaying higher efficacy due to production of more EEV as well as more total progeny virus.
Biodistribution of the viruses in naïve and immunized mice bearing MC38 PC
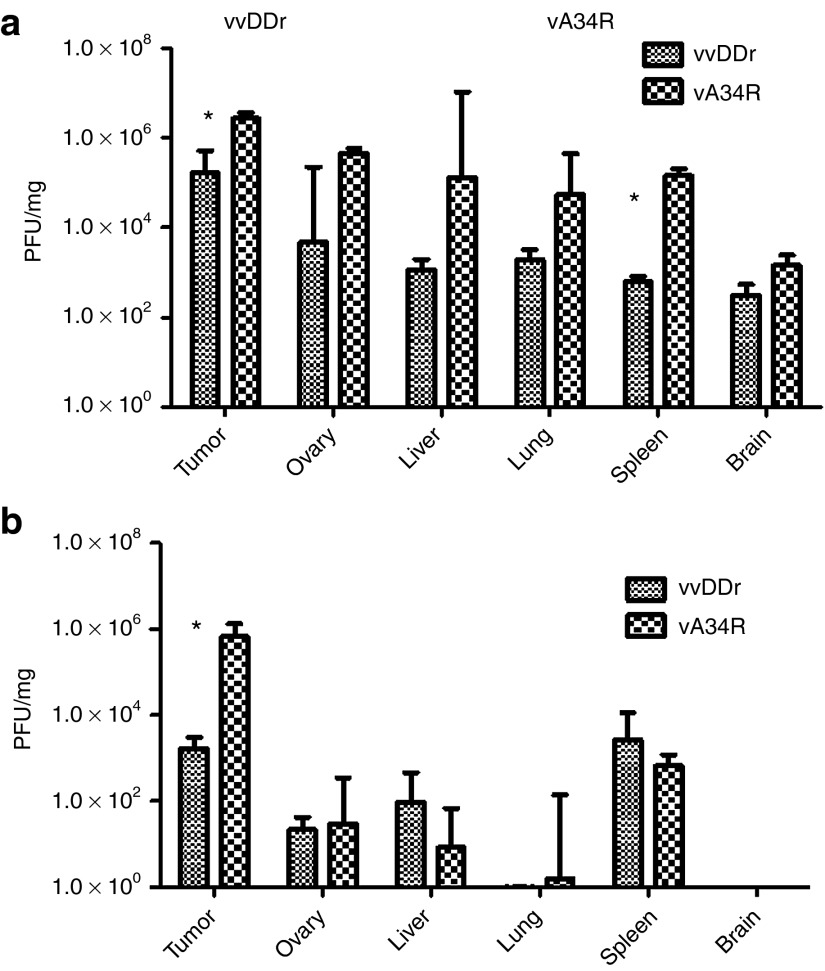
We studied the treatment effect and biodistribution of vA34R and vvDDr in naïve B6 mice bearing MC38 PC. In vivo imaging of PC on day 5 after viral treatment indicated a decrease in luciferase activity (implying less tumor burden) after vA34R treatment compared with vvDDr treatment (data not shown). Macroscopic tumor burden was nearly threefold lower after vA34R treatment compared with vvDDr treatment (mean weight in mg, 431 ± 10.8 versus 1,199 ± 103, P = 0.009). Tumor nodules and a panel of normal tissues (ovary, liver, lung, spleen, and brain) were then analyzed for viral titers (Figure 5a). In PC nodules (tumor), vA34R resulted in higher viral recovery compared with vvDDr (median pfu/mg protein, 2.71 × 106 versus 1.64 × 105, P < 0.05). However, vA34R was also recovered at higher levels in the normal tissues, although the differences were not statistically significant (with the exception of the spleen, P < 0.05).
Figure 5.
Biodistribution in naïve and preimmunized mice. (a) Biodistribution of both vA34R and vvDDr (1.0 × 108 pfu/ml, i.p.) in MC38 tumor bearing naïve mice was performed at day 4. Viral recovery was significantly higher in the vA34R-treated groups in both the tumor tissue (median pfu/ mg protein, 2.71 × 106 versus 1.64 × 105, P < 0.05) and spleen (median pfu/mg, 1.44 × 105 versus 6.3 × 102, P < 0.02) compared with vvDDr. (b) In preimmune mice, viruses were delivered in carrier cells (CC) for EEV production and release and biodistribution was analyzed on day 4. MC38 cancer cells infected at an MOI of 0.1 were used as carrier cells. A significant difference in the amount of viral recovery in tumor tissues was demonstrated (median pfu/mg, 6.66 × 105 for vA34R versus 2.13 × 103 for vvDDr, P = 0.03). *P < 0.05. MOI, multiplicity of infection; pfu, plaque-forming unit.
We then tested vA34R in the VV preimmunized B6 mice bearing MC38 PC. Immunization was performed with vA34R 30 days before tumor cell inoculation, which is associated with high levels of circulating antibodies.33 We initially used naked IMV forms of both viruses in preimmune PC-bearing mice and were able to recover little, if any, viruses from tumor tissues (data not shown). This is not surprising because our previous study demonstrated that delivery of vvDD to preimmunized mice yielded little recovery of virus from tumor unless immunosuppressive drugs were also applied.33 In that study, even the use of carrier cells yielded minimal viral recovery unless immunosuppressive drugs were applied. Here, we used cancer cells as carriers for delivering vA34R to assist in circumventing host antiviral immunity against the IMV form of the virus with the design that the EEV form of progeny virus will spread in and between tumor nodules without the need for immunosuppressive drugs. Preimmune mice with PC were treated with an i.p. injection of equivalent doses of carrier cells (MC38 cancer cells) loaded with either vvDDr or vA34R (at an MOI of 1.0). We have previously demonstrated that no cancer cells survive at this MOI.33 On day 5 after injection, mice were killed and all macroscopic tumors were harvested and analyzed for viral titers (Figure 5b). Median tissue viral recovery in the tumors was nearly 300 times higher with vA34R than with vvDDr (pfu/mg protein, 6.66 × 105 versus 2.13 × 103 pfu/mg, P = 0.03). However, for both vA34R and vvDDr, small and similar quantities of viruses were recovered from normal tissues. In comparison with the naïve mice, the amounts of viruses recovered from normal tissues had reduced dramatically (two to four logs of magnitude) (Figure 5), whereas recovery of A34R from tumor was nearly equivalent to the naïve mice.
Enhanced efficacy and host survival after vA34R treatment in naïve and preimmunized mice bearing MC38 PC
Naïve B6 mice bearing 7-day-old PC were treated with naked viruses at 2.0 × 108 pfu per mouse and were imaged on day 16 for bioluminescence activity from MC38-luc tumor. vA34R-treated cohorts displayed a lesser bioluminescence corresponding to better tumor response than vvDDr and control cohorts (Figure 6a). Long-term survival of animals was closely monitored. The animals might die of progression of the disease or any animal in which abdominal girth exceeded 1.5× the original measurement was euthanized and recorded as a death. Survival analysis showed that vA34R-treated mice displayed significantly prolonged survival compared with mock-treated control mice (median survival, 48 versus 25 days, P < 0.001) and vvDDr-treated mice (median survival, 48 versus 32 days, P < 0.005) (Figure 6b).
Figure 6.
The efficacy and survival with naked virus treatment in naïve mice. (a) Naïve intraperitoneal MC38-luc tumor bearing mice were treated on day 7 with either vvDDr or vA34R i.p. and their survival was followed over time. Bioluminescent images taken on day 16 show a greater reduction in tumor burden in the vA34R-treated groups. (b) Kaplan–Meier survival analysis shows vA34R-treated mice displayed significantly prolonged survival compared with mock-treated control mice (median survival, 48 versus 25 days, P < 0.001) and vvDDr-treated mice (median survival, 48 versus 32 days, P < 0.005).
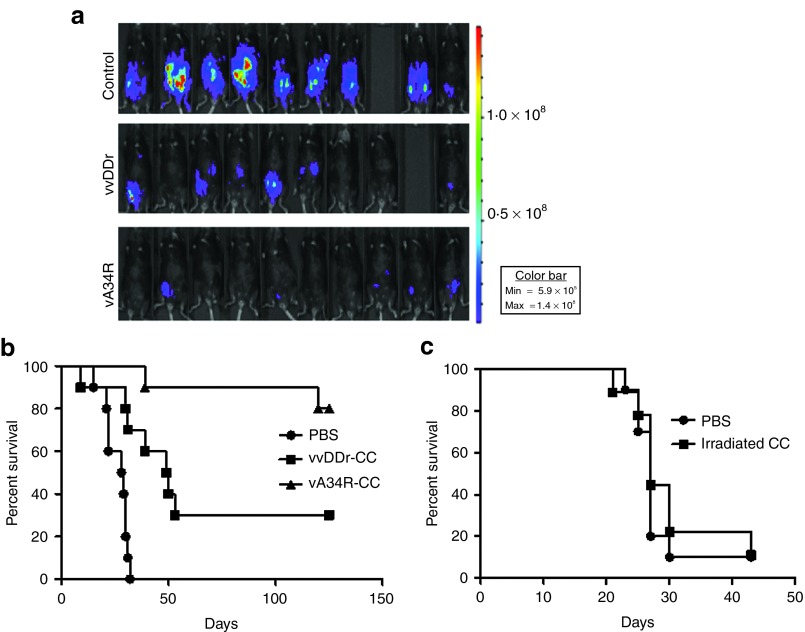
In preimmunized mice, we used carrier cell delivery of viruses for efficacy and survival studies as naked virus delivery was not able to achieve efficacy shown in our previous study.33 We have used 1.0 × 107 MC38 carrier cells infected at MOI of 1.0 for 12 hours. The carrier cells were injected i.p. into mice at day 7 after inoculation of i.p of MC38-luc cancer cells. Bioluminesence imaging at day 16 showed a better tumor response after cell-based delivery of vA34R than with cell-based delivery of vvDDr (Figure 7a). Survival of these animals was monitored and recorded. Survival analysis indicated that cell-based delivery of vA34R resulted in a significant improvement in survival compared with cell-based delivery of vvDDr (median survival, >125 versus 49.5 days, P = 0.017) or mock treatment (median survival, >125 versus 23.5 days, P < 0.001) (Figure 7b). In a separate experiment, we have examined the effect of irradiated MC38 carrier cells (Figure 7c). There was no effect of irradiated carrier cells on survival of MC38 PC-bearing animals in comparison with those treated with phosphate-buffered saline. These experiments suggested that cell-based delivery of vA34R could successfully overcome pre-existing antipoxvirus immunity, reaching high titers in target tumor tissues, and prolonging survival. Seven out of 10 mice treated with vA34R showed no residual viable tumor upon necropsy at time of sacrifice (125 days after tumor cell inoculation) suggesting complete remission in those mice.
Figure 7.
The efficacy and survival of vA34R treatment in preimmunized mice with MC38 PC. (a) Preimmunized mice bearing MC38-luc PC were treated with a single intraperitoneal injection of 1 × 107 pfu of either vvDDr or vA34R by cell-based delivery 7 days after tumor inoculation. Bioluminescent images taken on day 16 show a greater reduction in tumor burden in the vA34R-treated groups. (n = 9 for control and vvDDr groups; n = 10 for vA34R group). (b) Survival analysis shows the vA34R cohort survived longer than the vvDDr cohort (median survival, >125 versus 49.5 days, P = 0.017) and control cohort (median survival, >125 versus 23.5 days, P < 0.001). *P < 0.05. (c) Survival analysis of preimmunized mice with MC38 PC treated with PBS or irradiated MC38 carrier cells. P = 0.41 between the two groups. CC, carrier cells; PBS, phosphate-buffered saline; pfu, plaque-forming unit.
Although the tumor response in preimmunized mice with cell-based delivery of vA34R was far better than “naked” vA34R in naïve mice, different doses of virus were used and there are effects of pre-existing antipoxviral immunity. Thus, these two experiments should not be compared with each other (Figures 6b and 7b).
Discussion
In this study, we have demonstrated that a single pointed mutation in A34R in VV (vA34R) produces not only more EEV, but also more total infectious progeny virus, resulting in higher cytotoxicity to tumor cells. The enhanced spread to remote sites has been demonstrated in vitro with or without the neutralizing antibodies, and in vivo in MC38 tumor models resulting from MC38 cancer cells infected ex vivo at very low MOIs in both naïve and prevaccinated mice. We have shown that i.p. delivery of vA34R resulted in higher viral titers compared with vvDDr from tumor nodules in both naïve and preimmunized hosts. In the MC38 PC model in naïve mice, more infectious virus was recovered from vA34R than vvDDr in the tumor. This may explain the better therapeutic effect of vA34R. As a side effect, more vA34R virus was also recovered from some normal tissues (such as spleen). This is not too surprising because vA34R virus replicated and spread more efficiently in tumor cells than the control vvDDr, thus constantly barraging normal tissues with live virus. Despite this effect, the animals did not experience any notable pathogenicity or lethality from the virus.
More interesting results were obtained from the PC model in the preimmunized mice. In that model, we observed the same high recovery of vA34R in the tumor nodules as in those of naïve mice, but much lower amounts of virus from normal tissues. As for vvDDr, the yield of the progeny virus in the tumor nodules was two logs lower than vA34R, albeit also lower yields of the virus in normal tissues. Thus, the higher amount of viral delivery via carrier cells and higher viral replication of vA34R virus in the tumor in preimmunized mice has translated into an enhanced therapeutic effect.
Although viral delivery via a cell carrier is undoubtedly a more efficient way to deliver virus, we observed better long term survival in preimmunized mice than naïve mice. Many explanations for this observation exist. The carrier cell-mediated delivery of the OVs in preimmunized mice may be a more efficient and targeted approach to treat PC. Also, it may be that carrier cells serve as a virus factory and thus effectively increasing the virus dose delivered. In this context, vA34R replicates better than vvDDr and thus a higher dose of virus is delivered via cell carriers. The most obvious explanation could be that pre-existing immunity against the virus and tumor-antigen presentation by the carrier cells may boost stronger antitumor immune responses. Our previous work, however, suggested that our best antitumor effect in preimmune mice was with carrier cell delivery and immunosuppression, suggesting that virus survival and replication is more important than the immune response.
vA34R is an ideal oncolytic agent for PC
OVs have been explored previously for the treatment of PC in animal models.29,30,31,32,33 However, the utility of OVs, especially VV, has been associated with several problems in the context of PC treatment. First, access to these peritoneum-based nodules is restricted after systemic delivery of OVs due to the relative avascularity of the nodules and the existence of a peritoneal-plasma barrier.39 Second, the nodules are often too numerous, too small and in surgically inaccessible areas, making intratumoral injection of each of these nodules impossible. As VV typically spreads from cell to cell in a contiguous fashion, it is unlikely that diffuse spread will occur throughout the peritoneal cavity.33 Lastly, immunity to this highly immunogenic virus has thwarted any sustained oncolysis resulting in tumor cell division outpacing viral replication.33 In essence, there is an urgent need for a more efficient, rapidly replicating, yet tumor-selective virus that is capable of spreading from one nodule to another autonomously while evading the host antiviral immunity and ultimately leading to a reduction in tumor burden or complete tumor regression.
The new virus vA34R can function as such a virus for the treatment of PC. This EEV-enhanced virus enables free spread of virions inside the abdominal cavity. We circumvented the potential for increased toxicity due to enhanced spread by engineering the virus on the backbone of vvDD for tumor-selective viral replication. As EEVs cannot be easily isolated or purified, the administration of vA34R was in its IMV form, with the hope that EEVs would be produced in the targeted cancer cells in vivo. However, the host immune response directed against the immunogenic virus could prevent infection of the cancer cells by the virus, let alone the production of progeny virus (EEV and IMV forms) in the tumor tissue.33 Thus, the use of carrier cells to circumvent the immune barrier has been attempted with several OVs albeit with limited success.3,40,41 In the case of oncolytic VV, one reason for limited success may be that the cell carriers do not release virus efficiently in the absence of an EEV enhancing mutation. Any benefit from the initial stealth effect provided by cell-based delivery is annulled by the immune response targeted at poxvirus virions when they exit the carrier cells to infect the target tumor cells.33 It is only by delivering an EEV-enhanced poxvirus in carrier cells that efficacious virotherapy has been achieved.
Apart from aiding in stealth delivery, carrier cells possess additional theoretical benefits, such as tumor homing and amplification of the OV.40,41 We chose cancer cells as carrier cells for multiple reasons (i) vA34R was designed to be tumor specific; and (ii) the fate of tumor cells released into the peritoneal cavity is not random.42 Cancer cells often track to certain areas (omenta, hepatoduodenal ligament, right subdiaphragm, paracolic gutters, pouch of Douglas and relatively immobile intestinal loops) more commonly than others resulting in definite patterns of carcinomatosis.37 Cells tend to clog the channels of peritoneal fluid drainage (a function of cell size), resulting in nodules and ascites.42 Use of the same cancer cells would theoretically follow the same flow and homing pattern to deliver the virus at the high-yield locations.41,43 Syngeneic cancer cells belonging to the same cell line responsible for initial PC were used to simulate the clinical possibility of using the patients' own peritoneal cancer cells (obtained via biopsy) for cell-based delivery. The virally infected cancer cells as carrier cells has been shown to be safe in our previous study with no escape of live cancer cells that could lead to an increase in tumor burden.33 Further precautions such as the introduction of suicide genes into the virus or irradiation of carrier cells may be used in the clinical setting to ensure additional safety.
Oncolytic effect versus immune effect
Although the current study was not designed to elucidate the antitumor immunologic effect of this therapy, it is impossible to ignore this aspect of viral therapy, especially when utilizing a known, strong immunogen like VV. Also, as discussed above, the preimmune mice had an excellent response to therapy with vA34R suggesting an immune boost after prevaccination. Our previous work has demonstrated an improved antitumor response in nude mice compared with immune competent mice, suggesting that early clearance of the replicating virus in the immune competent host was detrimental to the antitumor response.8,32 Thus, we believe that the oncolytic effect for VV is more potent than its immune adjuvant effect in our animal models. Nevertheless, the concept of using OVs as adjuvants for enhanced tumor immunogenicity has been known and well studied over the last few decades.44 It was concluded that the immunogenicity of certain host cell components was greatly increased by incorporation of antigens into the makeup of the OV. Recently, Fang and collaborators have demonstrated that pre-existing adaptive immunity against an oncolytic adenovirus or poxvirus and/or a model tumor antigen could be redirected to tumor when injected with an OV with or without expression of a tumor antigen.45 Bridle et al. have also shown the benefits of a combined strategy wherein the immune response against an OV leads to enhanced therapeutic outcomes.46 In that strategy, their combination approach shifted the immune response from viral antigens to tumor antigens and further reduced the replication of virus in normal tissues, leading to enhancements in both efficacy and safety. Now, many investigators have considered the OV as a form of immunotherapy.47 The potency of this approach can be further enhanced in combination with other immunological regimens such as an agonist antibody specific for the costimulatory molecule 4-1BB.48 We have previously examined an OV expressing chemokines with the goal of enhancing the trafficking of tumor directed T cells into the tumor microenvironment.49 As we have demonstrated efficient uptake and spread of the A34R virus into tumor nodules in the peritoneum, we can consider incorporating and delivering genes for tumor antigens, cytokines, and/or chemokines to further enhance the therapeutic effect.
In summary, we have rationally designed a new immune-evasive and tumor-selective replicating oncolytic poxvirus that produces more total infectious virions, and spreads across distances more efficiently in the peritoneal cavity. It worked effectively not only in a PC model in naïve mice, but also in the preimmunized host where the pre-existing antiviral immunity may boost antitumor immunity and also clear the virus from normal tissues more effectively. Thus, this new virus may be an effective oncolytic agent for various types of patients with peritoneal cancer who may or may not have previous exposure to smallpox vaccine.
Materials and Methods
Cell lines. Mouse colon cancer MC38 cells and African green monkey kidney CV-1 cells have been used frequently in this laboratory. Firefly luciferase gene-tagged to MC38 (MC38-luc) cells, made by transduction with a firefly luciferase gene-coding lentivirus, were applied for IVIS bioluminescence imaging as described previously.33 AB12 is a murine mesothelioma cell line (a kind gift of Steven M Albelda at the University of Pennsylvania). MCF7 is a human breast cancer cell line. All cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin/streptomycin (Invitrogen, Carlsbad, CA).
Recombinant VV. Recombinant viruses used in this study were all the WR strain derivatives. vvDD-CD, a double viral gene-deleted (tk- and vgf-) VV armed with yeast cytosine deaminase gene,32 was used as the parental virus for homologous recombination. The shuttle plasmids (pA34Rm-DsRed, pA34Rwt-DsRed) were created that allowed for homologous recombination into the A34R locus of vvDD-CD, creating the new vA34R and vvDDr, respectively. Briefly, PCR fragments of A34R gene with or without K (151)→E point mutation were used as the left flank and A35R gene including its promoter was used as the right flank of the shuttle plasmid for homologous recombination. The DsRed gene driven by pSE/L promoter was cloned between the two flanks for use as a selection marker for the new vvDDr and vA34R. To make the new viruses, CV-1 cells were infected with vvDD-CD and then transfected with pA34Rm-DsRed for vA34R, pA34Rwt-DsRed for vvDDr. Selection of the new recombinant viruses was based on expression of DsRED fluorescence in resulting plaques. After three rounds of plaque purification in 96-well plates, viral DNA from individual plaques was extracted and sequenced to confirm the presence of the correct point mutation.
MTS cytotoxicity assay in vitro. For viral cytotoxicity assay, cancer cells were plated at 1.0 × 104 cells per well in 96-well plates for growth overnight, and then subsequently infected with viruses at MOIs of 0.01, 0.05, 0.1, 0.5, 1.0, and 5.0. Cell viability was determined at 48 and 72 hours after infection, by CellTiter 96 Aqueous Nonradioactive Cell Proliferation Assay, sometimes called MTS assay (Promega, Madison, MI).
Viral replication assays in vitro. Viral replication assays were performed as previously described.18,27 Briefly, 1.0 × 105 MC38-luc cells, in six-well plates were infected with vA34R and vvDDr at MOIs of 0.1, 1.0, or 10 in 1 ml of 2% fetal bovine serum-containing–Dulbecco's modified Eagle's medium for 2 hours at 37 °C. Following infection, cells were cultured in Dulbecco's modified Eagle's medium-10% fetal bovine serum until harvesting at 12, 24, 48, and 72 hours after viral infection. The cell pellet was homogenized using a FastPrep Cell Disrupter (Model FP120; Qbiogene, Carlsbad, CA) to release virions, and the resulting cell lysates were titered on CV-1 cells to determine viral load.
Transwell assays and fluorescence-activated cell sorting. Transwell Permeable Supports with 3 µm polycarbonate transmembrane pores six-well transwells (Corning, Corning, NY) were used. 1.0 × 105 MC38 cells/well were plated in receiver (bottom) plates and 1.0 × 104 MC38 cells were plated in each insert well. vA34R and vvDDr at MOI of 1.0 was used to infect the cells in bottom wells, and the expression of DsRed florescence in transwell inserts was monitored over time. At 2 hours after infection, anti-VIG from Centers for Disease Control and Prevention at dilution of 1:1,000 was added to the growth medium. Procedures for cultivation and harvest of cells were performed according to the manufacturer's instructions. Flow cytometry for red fluorescent protein (DsRed)-positive cells were performed with cells from the insert wells at 72 hours after infection.
Infected cancer cells as carrier cells and evaluation of infectivity. MC38 cancer cells were infected in vitro with either vA34R or vvDDr at an MOI of 1.0. At 12 hours after infection, cells were harvested, washed in cold 1× phosphate-buffered saline three times, counted and injected i.p. into MC38-luc PC-bearing mice. As a control, MC38 cells were irradiated (20 Gy), washed and injected into mice. The irradiated MC38 cells do not proliferate but survived up to 2–3 days in cell culture.
To evaluate the infectivity of the viruses for MC38 cancer cells, cells infected with virus at various MOIs were harvested at 3, 6, or 12 hours after infection. These cells were randomly sorted by flow sorting one cell per well onto CV-1 cells in 96-well plates. The expression of red fluorescent protein (DsRed) at 48 hours in CV-1 cells serves as good indicator of the original targeted cells being infected.
Mice. Female C57BL/6 mice were obtained from Taconic Farms (Germantown, NY). All animal studies conducted at the two institutions were approved by the Institutional Animal Care and Use Committees.
VV immunization, PC model establishment, and living animal imaging. VV immunization was performed by injecting vA34R at 4.0 × 106 pfu per mouse i.p., which generate high levels of adaptive immunity as we have shown previously.33 One month after viral immunization, mice were used for tumor cell inoculation and additional experiments. The PC tumor model was established by injection of 5.0 × 105 or 1.0 × 106 MC38-luc cells i.p. Tumor establishment was monitored by live animal IVIS imaging. All viral injections were given i.p. on days 5 or 7 after tumor inoculation.
The in vivo optical imaging in living animals was performed using a Xenogen IVIS 200 Optical In Vivo Imaging System (Caliper Life Sciences, Hopkinton, MA), with technical assistance from the Small Animal Imaging Core Facility of the University of Pittsburgh Cancer Institute.
Long-term survival of mice. The health and survival of treated mice was closely monitored. All mice subjected to peritoneal tumors were monitored via caliper measurements for changes in abdominal girth. There are two criteria for death of animals: natural death due to the disease or any animal in which abdominal girth exceeded 1.5× the original measurement was euthanized and recorded as a death.
Statistical analysis. Raw data were recorded electronically and statistical analyses were performed with Microsoft Excel or the SPSS Software version 18 (IBM, New York, NY). Central tendencies were expressed either as means ± SD with Student's t-test or medians with Kruskal–Wallis as appropriate. An α value (P) of 0.05 was considered as statistically significant, and all P values were two sided.
SUPPLEMENTARY MATERIAL Figure S1. Schematic presentation of vA34R, vvDDr, and parental virus vvDD. Figure S2. Infectivity, yield of the viruses and cytotoxicity in MC38 cancer cells and CV-1 cells. Figure S3. The infectivity of vvDDr and vA34R in CV-1 cells (a) and MC38 cancer cells (b) at higher MOIs.
Acknowledgments
This work is supported by NIH grant R01CA155925 (to D.L.B.) and by David C Koch Regional Therapy Cancer Center. P.T. was supported by American College of Surgeons Resident Research Scholarship, and M.S. and F.A. by NIH training grant T32CA113263-01. This project used University of Pittsburgh Cancer Institute shared resources that are supported in part by NIH grant award P30CA047904. D.L.B. serves as a member of the Scientific Advisory Board, and D.L.B. and S.H.T. are shareholders of Jennerex BioTherapeutics, a company focusing on development of oncolytic viruses. D.L.B. receives patent royalties on oncolytic vaccinia virus. The other authors declared no conflict of interest.
Supplementary Material
References
- Bourke MG, Salwa S, Harrington KJ, Kucharczyk MJ, Forde PF, de Kruijf M.et al. (2011The emerging role of viruses in the treatment of solid tumours Cancer Treat Rev 37618–632. [DOI] [PubMed] [Google Scholar]
- Stanford MM, Bell JC., and, Vähä-Koskela MJ. Novel oncolytic viruses: riding high on the next wave. Cytokine Growth Factor Rev. 2010;21:177–183. doi: 10.1016/j.cytogfr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Thorne SH., and, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doceul V, Hollinshead M, van der Linden L., and, Smith GL. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science. 2010;327:873–876. doi: 10.1126/science.1183173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL., and, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16:472–479. doi: 10.1016/j.tim.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK.et al. (2001Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes Cancer Res 618751–8757. [PubMed] [Google Scholar]
- Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y.et al. (2005The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2 Cancer Res 659991–9998. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ.et al. (2007Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus Cancer Res 6710038–10046. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Liang W, Contag CH., and, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- Sathaiah M, Thirunavukkarasu P, O'Malley ME, Kavanagh MA, Ravindranathan R, Austin F.et al. (2012Oncolytic poxvirus armed with Fas ligand leads to induction of cellular Fas receptor and selective viral replication in FasR-negative cancer Cancer Gene Ther 19192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]
- Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, Ilkow C.et al. (2012The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers Mol Ther 20749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR.et al. (2000Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant Cancer Gene Ther 766–73. [DOI] [PubMed] [Google Scholar]
- Buller RM, Chakrabarti S, Moss B., and, Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988;164:182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Blasco R, Sisler JR., and, Moss B. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J Virol. 1993;67:3319–3325. doi: 10.1128/jvi.67.6.3319-3325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Murphy BJ., and, Law M. Vaccinia virus motility. Annu Rev Microbiol. 2003;57:323–342. doi: 10.1146/annurev.micro.57.030502.091037. [DOI] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A., and, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83 Pt 12:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- McIntosh AA., and, Smith GL. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J Virol. 1996;70:272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R.et al. (2000Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV Mol Biol Cell 112497–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL., and, Vanderplasschen A. Extracellular enveloped vaccinia virus. Entry, egress, and evasion. Adv Exp Med Biol. 1998;440:395–414. [PubMed] [Google Scholar]
- Vanderplasschen A, Mathew E, Hollinshead M, Sim RB., and, Smith GL. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci USA. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplasschen A, Hollinshead M., and, Smith GL. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J Gen Virol. 1998;79 (Pt 4):877–887. doi: 10.1099/0022-1317-79-4-877. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen A., and, Smith GL. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne LG. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Krauss O, Hollinshead R, Hollinshead M., and, Smith GL. An investigation of incorporation of cellular antigens into vaccinia virus particles. J Gen Virol. 2002;83 Pt 10:2347–2359. doi: 10.1099/0022-1317-83-10-2347. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y. Extracellular enveloped vaccinia virus escapes neutralization. Virology. 1996;217:478–485. doi: 10.1006/viro.1996.0142. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen A, Hollinshead M., and, Smith GL. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J Gen Virol. 1997;78 (Pt 8):2041–2048. doi: 10.1099/0022-1317-78-8-2041. [DOI] [PubMed] [Google Scholar]
- Coukos G, Makrigiannakis A, Kang EH, Caparelli D, Benjamin I, Kaiser LR.et al. (1999Use of carrier cells to deliver a replication-selective herpes simplex virus-1 mutant for the intraperitoneal therapy of epithelial ovarian cancer Clin Cancer Res 51523–1537. [PubMed] [Google Scholar]
- Wildner O., and, Morris JC. Therapy of peritoneal carcinomatosis from colon cancer with oncolytic adenoviruses. J Gene Med. 2000;2:353–360. doi: 10.1002/1521-2254(200009/10)2:5<353::AID-JGM130>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J.et al. (2002Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer Cancer Gene Ther 9935–945. [DOI] [PubMed] [Google Scholar]
- Chalikonda S, Kivlen MH, O'Malley ME, Eric Dong XD, McCart JA, Gorry MC.et al. (2008Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene Cancer Gene Ther 15115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZS, Parimi V, O'Malley ME, Thirunavukarasu P, Sathaiah M, Austin F.et al. (2010The combination of immunosuppression and carrier cells significantly enhances the efficacy of oncolytic poxvirus in the pre-immunized host Gene Ther 171465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J.et al. (2000Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study Cancer 88358–363. [DOI] [PubMed] [Google Scholar]
- Koppe MJ, Boerman OC, Oyen WJ., and, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao CQ, Yan TD, Liauw W., and, Morris DL. Comparison of optimally resected hepatectomy and peritonectomy patients with colorectal cancer metastasis. J Surg Oncol. 2009;100:529–533. doi: 10.1002/jso.21369. [DOI] [PubMed] [Google Scholar]
- Davies JM., and, O'Neil B. Peritoneal carcinomatosis of gastrointestinal origin: natural history and treatment options. Expert Opin Investig Drugs. 2009;18:913–919. doi: 10.1517/13543780902939151. [DOI] [PubMed] [Google Scholar]
- Piso P, Glockzin G, von Breitenbuch P, Popp FC, Dahlke MH, Schlitt HJ.et al. (2009Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies J Surg Oncol 100317–320. [DOI] [PubMed] [Google Scholar]
- Jacquet P., and, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- Raykov Z., and, Rommelaere J. Potential of tumour cells for delivering oncolytic viruses. Gene Ther. 2008;15:704–710. doi: 10.1038/gt.2008.34. [DOI] [PubMed] [Google Scholar]
- Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A., and, Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusamura S, Baratti D, Zaffaroni N, Villa R, Laterza B, Balestra MR.et al. (2010Pathophysiology and biology of peritoneal carcinomatosis World J Gastrointest Oncol 212–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Nawa A, Luo C, Kamakura M, Goshima F, Kondo C.et al. (2011Carrier cell-based delivery of replication-competent HSV-1 mutants enhances antitumor effect for ovarian cancer Cancer Gene Ther 1877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmann J., and, Klein PA. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967;126:93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Davis JJ, Zhu H, Dong F, Guo W, Ang J.et al. (2007Redirecting adaptive immunity against foreign antigens to tumors for cancer therapy Cancer Biol Ther 61773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E.et al. (2010Potentiating cancer immunotherapy using an oncolytic virus Mol Ther 181430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA., and, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP.et al. (2012Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer Cancer Res 721651–1660. [DOI] [PubMed] [Google Scholar]
- Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P.et al. (2011Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer Mol Ther 19650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.