Fig. 3.
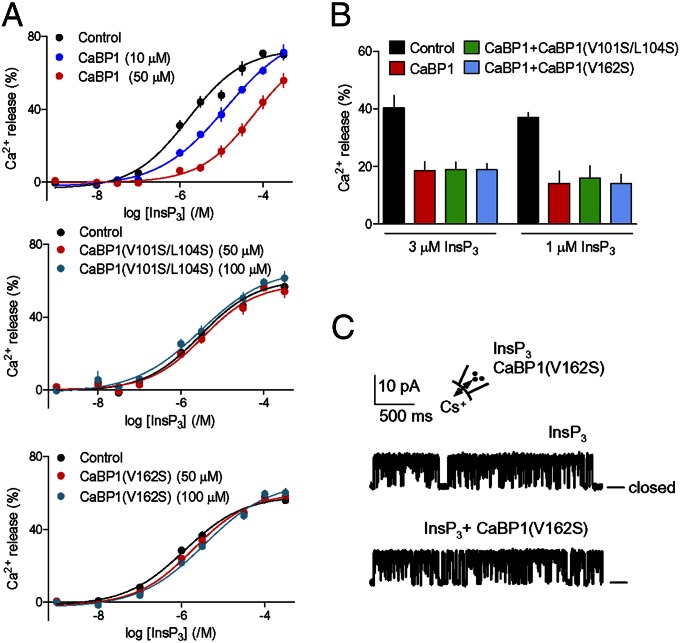
Hydrophobic residues in CaBP1 are essential for inhibition of InsP3R. (A) Inhibition of InsP3-evoked Ca2+ release by CaBP1 is abolished after mutation of its key hydrophobic residues. Permeabilized DT40–InsP3R1 cells in CLM with [Ca2+]c of 3.5 μM were incubated with the indicated concentrations of CaBP1, CaBP1(V162S), or CaBP1(V101S/L104S) (10 min) before adding InsP3. Results show the concentration-dependent release of Ca2+ by InsP3. (B) Ca2+ release evoked by 1 or 3 μM InsP3 alone, with CaBP1 (50 μM), or with CaBP1 (50 μM) and mutant CaBP1 (100 μM). Results (A and B) are means ± SEM; n = 4, with duplicate determinations in each. Similar results performed in CLM with 1.2 μM [Ca2+]c are shown in Fig. S5 D and E. Summary results are in Table S3. (C) Typical patch-clamp recordings from single InsP3R1 in medium with [Ca2+]c of 1.5 μM stimulated with InsP3 (10 μM) alone or in combination with CaBP1(V162S) (10 μM) shows that the mutant CaBP1 is inactive. Bars show the closed state. The holding potential was +40 mV. Summary results are shown in Fig. S1F. Fig. 2A shows the positions of mutated residues.