Fig. 4.
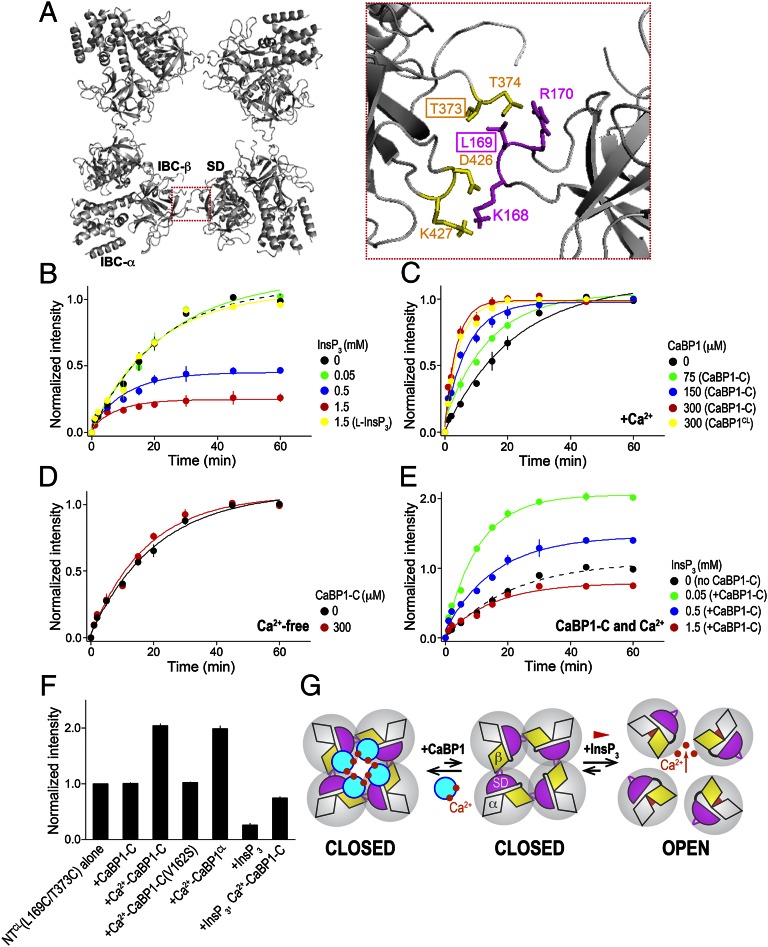
Opposing effects of CaBP1 and InsP3 on interactions between NTs. (A) Top view of the tetrameric structure of NTs, and close-up view of the boxed area of the intersubunit interface between the HS loop of the SD (magenta) and IBC-β (yellow) (29). The two residues that were replaced by Cys for CuP cross-linking analyses are boxed. (B) InsP3 weakens the interactions between NT subunits and thereby the rate of CuP-mediated cross-linking of tetrameric NT. NTCL(L169C/T373C) (75 μM) in medium containing 5 mM CaCl2 was incubated with CuP (100 μM) alone or with the indicated concentrations of InsP3 (the naturally occurring d-isomer) or biologically inactive l-InsP3. Results are expressed as fractions of the average intensity of the tetramer band detected at 60 min in the control incubation (no InsP3). The results with NTCL(L169C/T373C) alone (black) are shown for comparison. (C and D) Similar cross-linking experiments show that CaBP1 has the opposite effect to InsP3. Effects on tetramer formation of the indicated concentrations of the C lobe of CaBP1 (CaBP-C) and of the Cys-less form of full-length CaBP1 (CaBP1CL) in medium containing 5 mM CaCl2 (C) or in Ca2+-free medium (D). (E) The C-terminal of CaBP1 substantially blocks the destabilization of NT subunit interactions by InsP3. Effects of the indicated concentrations of InsP3 with CaBP1 C lobe (300 μM) in medium containing 5 mM CaCl2. Results are expressed as fractions of the average intensity of the tetramer band detected at 60 min in the control incubation (no InsP3 or CaBP1). The results with NTCL(L169C/T373C) alone (black) are shown for comparison. (F) Summary data show amounts of cross-linked NTCL(L169C/T373C) tetramer detected at 60 min relative to NTCL(L169C/T373C) alone at 60 min. Results in B–F show means ± standard deviation from three independent experiments. InsP3 denotes d-InsP3 unless indicated otherwise. The data from which these analyses derive are shown in Fig. S6, and the rate constants and normalized band intensities in Table S4. (G) Interactions between adjacent NTs mediated by IBC-β (yellow) and the HS loop of the SD (magenta) hold the tetrameric InsP3R in a closed state. InsP3 binding closes the clam-like IBC, disrupting these intersubunit interactions, and allowing the channel to open. The cytosolic vestibule of the InsP3R with four CaBP1s (cyan) bound is probably at least 5 Å across and unlikely to impede the flow of ions. Instead, we suggest that CaBP1 clamps the intersubunit interactions and thereby inhibits channel opening.