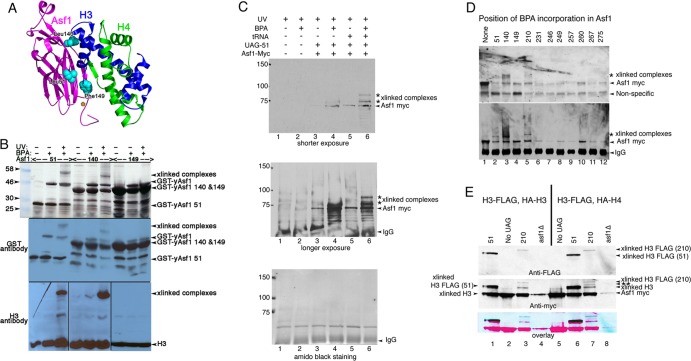
Fig 2.
Asf1 residue 210 with substitutions of BPA cross-links to histone H3. (A) Ribbon diagram of yAsf1 (aa 1 to 164) with H3 and H4 showing the proximity of yAsf1 residues Leu51, Leu140, and Phe149 (cyan) to H3. yAsf1 Trp153 is marked with an orange star. (B) Silver-stained gel (top) and Western blot analyses showing production of BPA-containing yAsf1 and cross-linking of Asf1-BPA-51 and Asf1-BPA-140 to histone H3 with UV treatment. E. coli transformed with a triple yAsf1-H3/H4 expression vector that harbors amber stop codons at position 51, 140, or 149 produced truncated yAsf1 in the absence of BPA. In the presence of BPA, full-length yAsf1 was observed. With UV treatment, cross-linked (xlinked) complexes that contain both yAsf1 and H3 appear. (C) yAsf1-Myc displays altered mobility (marked with an asterisk) only in the presence of photoactivatable artificial amino acid, BPA, the amber suppressor tRNA plasmid, and the Asf1-L51-UAG plasmid in yeast. Anti-myc immunoblot analysis of immunoprecipitated samples: in lanes 1 and 2, BKD094 (asf1Δ pRS314); in lanes 3 and 4, MCY001 (asf1Δ pRS314-Asf1-UAG-51); and in lanes 5 and 6, BKD0237 (asf1Δ pRS314-Asf1-UAG-51, pLH157-KAN). (D) Replacement of yAsf1 residues 51, 140, and 210 with BPA leads to cross-linked protein species. (Top) Anti-myc immunoblot analysis of extracts. (Bottom) Immunoprecipitated samples. The strains are BKD257 (None), BKD237 (51), BKD259 (140), BKD263 (149), BKD241 (210), BKD243 (231), BKD245 (246), BKD247 (249), BKD249 (257), BKD251 (260), BKD261 (267), and BKD255 (275). (E) yAsf1 residues 210 and 51, when replaced with BPA, cross-link to C-terminally FLAG-tagged histone H3. (Top) Immunoprecipitated yAsf1-Myc immunoblotted with anti-FLAG Ab. (Middle) Analysis of the same blot after being stripped and reprobed with anti-Myc Ab. (Bottom) Overlay of the anti-FLAG and anti-Myc Ab images. The blue/black bands indicate overlap between the Myc and FLAG signals. The HA and FLAG tags are not distinguishable by their electrophoretic mobilities. The identity of the Asf1-cross-linked central band, marked with a double asterisk, has not been established. Lanes 1 to 4, FLAG-H3 plus HA-H4; lanes 5 to 8, FLAG-H3 plus HA-H3. The strains are as follows: (lane 1) BKD382 (40), (lane 2) BKD378 (No UAG), (lane 3) BKD386 (210), (lane 4) BKD390 (asf1Δ), (lane 5) BKD379 (No UAG), (lane 6) BKD383 (51), (lane 7) BKD387 (210), and (lane 8) BKD391 (no Asf1). The yAsf1 signals seen in lanes 4 and 8 are due to interwell sample leakage.