Fig 3.
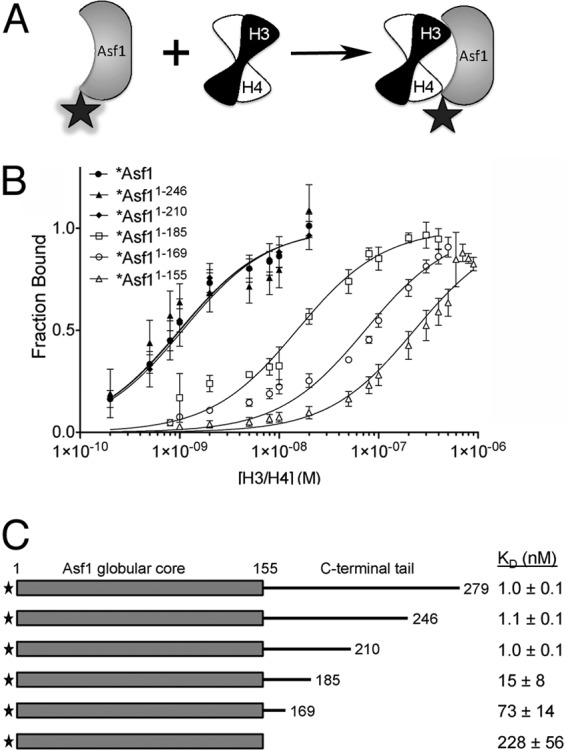
A specific region on the yAsf1 tail enhances binding affinity to H3/H4. (A) Asf1 proteins were fluorophore labeled at a position not directly involved in binding to H3/H4 dimers. The fluorophore (shaded star) fluoresces strongly in the free Asf1 protein. However, the binding of the H3/H4 dimer to Asf1 results in quenching of this fluorescence (plain star). (B) Analysis of fluorescence-quenching data for titration of histones H3/H4 into 1 nM Alexa Fluor 532-labeled full-length yAsf1 (1 to 279) and C-terminal truncations (1-246, 1-210, 1-185, 1-169, and 1-155). The data were fitted with a ligand-depleted binding model (equation 1) or single site-binding isotherm (equation 2), depending on the final KD values. The error bars indicate standard deviations from three independent experiments. (C) Table of KD values aligned with a diagram showing yAsf1 C-terminal tail truncations demonstrating that residues 156 to 209 in the C-terminal tail of yAsf1 contribute to the binding affinity of yAsf1 for H3/H4.
