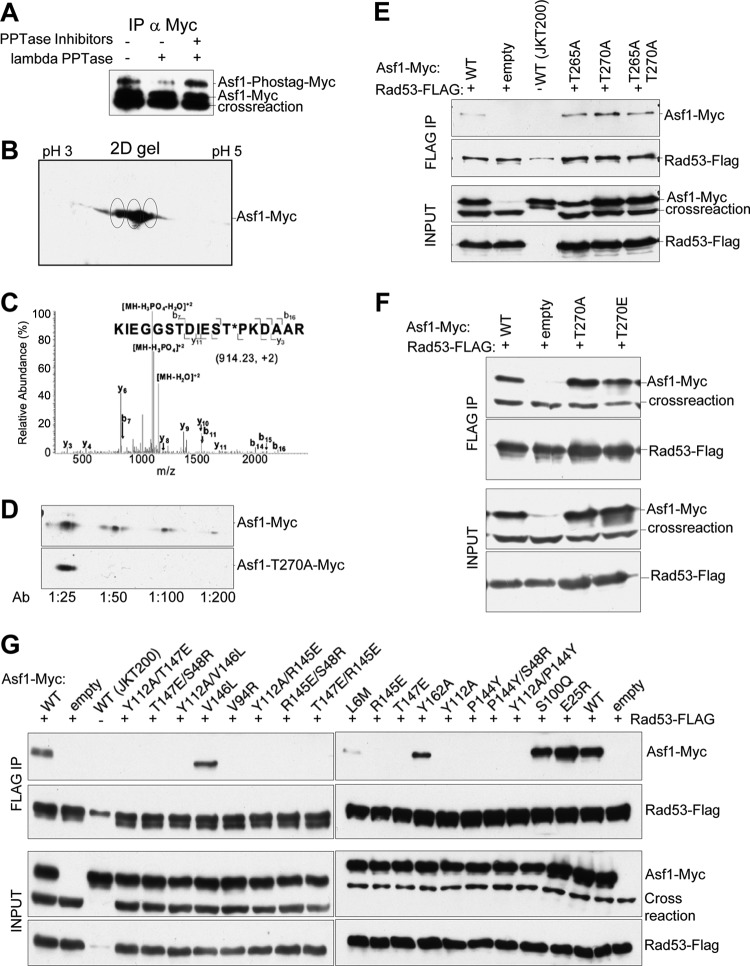
Fig 5.
Asf1 is phosphorylated on T270, and mutations in the Asf1-histone binding surface prevent Asf1-Rad53 coimmunoprecipitation. (A) yAsf1-Myc extracts from strain HZY1161, expressing FLAG-tagged Rad53 and containing pRS314-Asf1-Myc, were treated with and without λ phosphatase and phosphatase inhibitors. The immunoprecipitates were resolved by Mn2+ Phos-tag SDS-PAGE and analyzed by immunoblotting with anti-Myc antibodies. (B) Asf1 might be multiply phosphorylated. Shown is a 2D gel analysis of yAsf1-Myc immunoprecipitated from strain HZY1161 containing pRS314-Asf1-Myc and blotted with anti-Myc antibodies. Putative yAsf1 phosphorylation states are circled. (C) LC–MS-MS analysis of a tryptic digest of Asf1-myc immunoprecipitated from strain JKT200 carrying pRS314-Asf1-Myc. The assigned fragment ions are shown on the peptide sequence. (D) An affinity-purified antibody raised to phosphorylated yAsf1 T270 shows a signal on an immunoblot of whole-cell extracts of JKT200 with pRS314-Asf1-Myc, but not with pRS314-Asf1T270A-Myc, at dilutions from 1:50 to 1:200. (E and F) pRS314-Asf1-Myc plasmids or an empty-vector control (pRS314) was transformed into HZY1161 expressing FLAG-tagged Rad53. JKT0200 lacks ASF1 and FLAG-tagged Rad53. yAsf1-Myc was coimmunoprecipitated with Rad53-FLAG, and immoprecipitated samples were analyzed by immunoblotting. (E) Coimmunoprecipitation of WT or mutated yAsf1-Myc with Rad53-FLAG shows that mutations of yAsf1 T265 and/or T270 did not disrupt its association with Rad53. A cross-reacting protein that migrates more quickly than yAsf1-Myc (crossreaction), is detected by the anti-Myc Ab and is present in the absence of Asf1 (JKT0200). A more slowly migrating cross-reacting species is occasionally seen with the anti-FLAG Ab, and is present in the absence of Rad53-FLAG (JKT0200). (F) yAsf1 mutations to prevent or mimic phosphorylation of T270 do not abolish or enhance the association of yAsf1 and Rad53. (G) Asf1's histone binding region is important for Rad53 association. Coimmunoprecipitation of WT or mutated yAsf1-Myc with Rad53-FLAG was analyzed by immunoblotting.