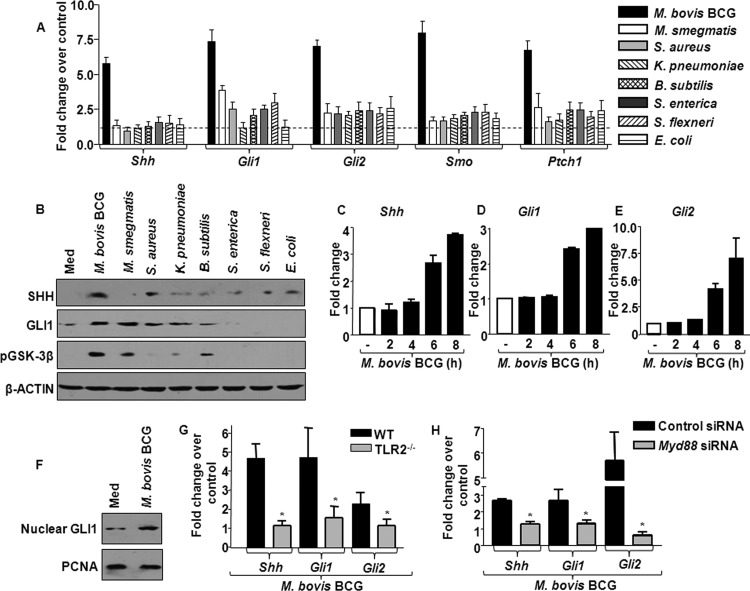
Fig 1.
Among the pathogens, M. bovis BCG predominantly upregulates SHH signaling through TLR2. (A) Transcript analysis of SHH signaling activation markers Shh, Gli1, Gli2, Smo, and Ptch1 upon infection of mouse peritoneal macrophages with various microbes (mean ± SE, n = 6). (B) Total protein levels of SHH, GLI1, and pGSK-3β were analyzed by immunoblotting. Blots are representative of 3 independent experiments with β-actin as a loading control. (C to E) Time-kinetic analysis of Shh (C), Gli1 (D), and Gli2 (E) assayed by quantitative real-time RT-PCR. (F) The activation status of SHH signaling was assessed by analyzing GLI1 in the nuclear fractions of macrophages infected with M. bovis BCG. (G and H) Expression of Shh, Gli1, and Gli2 was evaluated by quantitative real-time RT-PCR in WT or TLR2-null macrophages infected with M. bovis BCG (G) or in M. bovis BCG-infected RAW 264.7 macrophages transfected with control siRNA or Myd88 siRNA (H). Data represent means ± SEs (n = 3). *, P < 0.05 versus WT-infected macrophages or M. bovis BCG-infected control siRNA-transfected macrophages. TLR2−/−, TLR2 knockout; Med, medium.