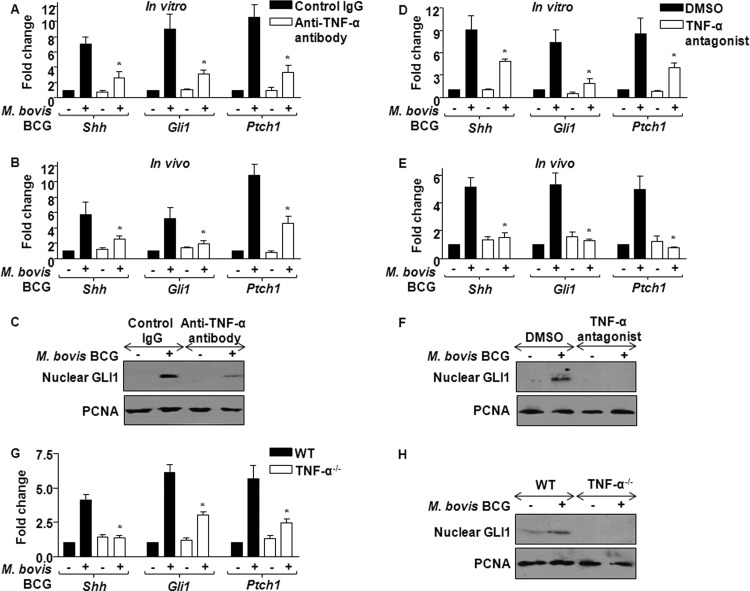
Fig 4.
TNF-α is a critical mediator of M. bovis BCG-trigged SHH signaling. (A) Macrophages were treated in vitro with anti-TNF-α antibody (10 μg) 6 h prior to M. bovis BCG infection, and SHH signaling pathway components were monitored by quantitative real-time RT-PCR. Data represent means ± SEs (n = 3). *, P < 0.05 versus control IgG-treated M. bovis BCG-infected macrophages. (B) Anti-TNF-α antibody (0.1 mg) was injected i.p. into each mouse for 12 h prior to i.p. infection with M. bovis BCG. Peritoneal macrophages isolated were analyzed for canonical SHH signaling molecules (mean ± SE, n = 3). *, P < 0.05 versus control IgG-injected M. bovis BCG-infected mice. (C) The activation status of SHH signaling was ascertained by nuclear translocation of GLI1 with or without in vitro treatment of anti-TNF-α antibody (10 μg). Blots are representative of 3 independent experiments. (D and E) Macrophages were treated in vitro with 5 μg (D) or mice were injected with 0.5 mg (E) of TNF-α antagonist, and M. bovis BCG-induced SHH signaling induction in peritoneal macrophages was monitored using quantitative real-time RT-PCR for Shh, Gli1, and Ptch1. Data represent means ± SEs (n = 3). *, P < 0.05 versus control IgG-treated M. bovis BCG-infected macrophages or mice. (F) GLI1 nuclear translocation was assayed in macrophages treated with TNF-α antagonist with immunoblotting. DMSO was utilized as the vehicle control. (G and H) M. bovis BCG-induced SHH signaling was analyzed in bone marrow-derived macrophages from TNF-α-null mice or WT mice using quantitative real-time RT-PCR (G) or immunoblotting (H). Data represent means ± SEs (n = 4). *, P < 0.05 versus M. bovis BCG-infected WT bone marrow-derived macrophages. Blots are representative of 3 independent experiments.