Figure 3.
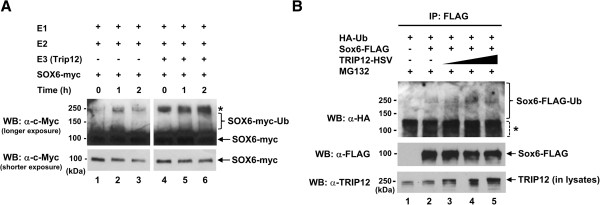
TRIP12 ubiquitinates Sox6 in vitro and in vivo. (A) In vitro ubiquitination of SOX6 protein was performed using purified SOX6-myc as the substrate and TRIP12 along with the combination of enzymes as indicated in the figure (see the Methods section for details). Lanes 4–6 contain 3 μg of TRIP12-HSV. Western blot (WB) was performed using c-Myc antibody to detect the degree of ubiquitination of Sox6-myc. Asterisk indicates non-specific bands detected in all reactions. (B) Sox6 is ubiquitinated by TRIP12 in vivo. HEK293 cells were cotransfected with plasmid DNAs encoding HA-Ub, Sox6-FLAG, and increasing amounts (0.5, 1, and 1.5 μg) of TRIP12-HSV, and lysates were immunoprecipitated (IP) with anti-DYKDDDDK (FLAG) antibody, and then processed for Western blotting (WB) using anti-HA antibody. Asterisk indicates non-specific bands detected in all IP samples, although it is possible that these bands also contain ubiquitinated Sox6-FLAG protein of lower molecular weights (with ~1 to 4 ubiquitin moieties) in Sox6-FLAG-transfected samples. The same membrane was subsequently incubated with anti-DYKDDDDK (FLAG) antibody to detect Sox6-FLAG protein; 10 μg (2%) of input protein samples (lysates) was also subjected to Western blotting using anti-TRIP 12 antibody to detect both endogenous TRIP12 and overexpressed TRIP12-HSV proteins.
