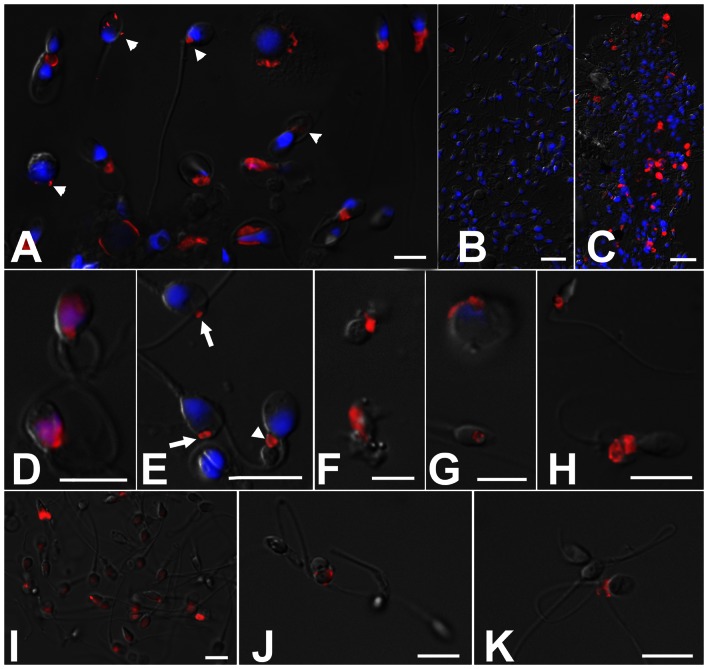
Figure 3. Epifluorescence microscopy of sperm samples processed for flow cytometric measurements of SPTRX3 (red).
The DNA-stain DAPI (blue) was added after flow cytometric analysis. Epifluorescence images are superimposed onto parfocal differential interference contrast (DIC) images (gray). (A) Examples of SPTRX3 labeling in samples from male infertility patients. Arrowheads point to minor carryovers of residual cytoplasm in spermatozoa and immature sperm forms; some of these cytoplasmic structures may not be detectable by conventional transmitted light microscopy, but can be identified accurately by SPTRX3 labeling. (B, C) The difference in SPTRX3 content is obvious even at low magnification between a sample from a presumed fertile donor (B) and the one of a patient previously diagnosed with oligo-astheno-teratozoospermia (C). (D–G) High magnification renderings of various patterns of SPTRX3 retention in defective spermatozoa, including a predominant sperm head/nuclear labeling (D), minor SPTRX3 retention in the sperm head (E; arrows) and sperm tail connecting piece (E; arrowhead), sperm-derived cellular debris free of DNA/chromatin (F), immature, spermatid-like form (G; top), and SPTRX3-filled large nuclear vacuole (G; bottom). (H) Immunolabeling of SPTRX3 in a frozen-thawed semen sample labeled according to a simplified protocol omitting sperm fixation and washing. (I–K) Detection of SPTRX3 before (I) and after semen purification on PureSperm gradient (J), and in a sample purified by swim-up after PureSperm treatment (K). Defective spermatozoa are not removed completely by this two-step procedure. Scale bars are 10 µm in all panels except B, C, where they represent 20 µm.