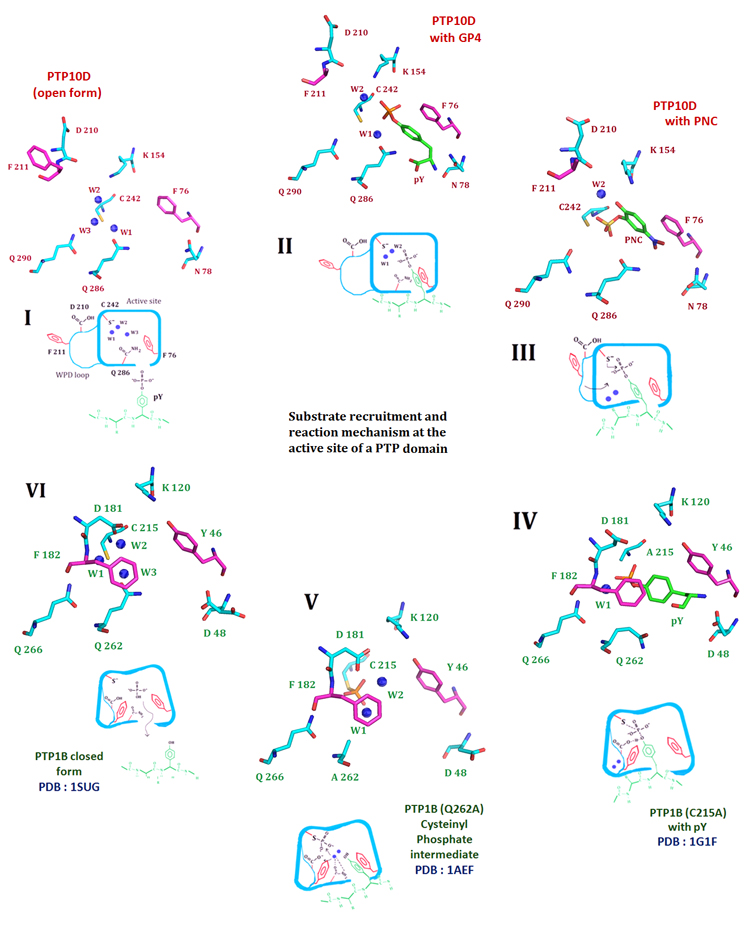
Figure 2. The crystal structure of PTP10D offers mechanistic insights into distinct steps of the phosphatase reaction.
I. The de-protonated active site cysteine prior to substrate entry into the active site. Three conserved water molecules hydrate the active site of PTP10D (PTP10D open structure). II. Substrate entry into the active site is mediated by Phe76 of the KNRY loop (based on the structure of PTP10D with the GP4 peptide). The incoming substrate displaces water W3. III. The substrate approaches the active site cysteine by displacing water W1. Aryl stacking interactions with Phe76 maintains the optimum conformation of the incoming substrate (based on the crystal structure of PTP10D with PNC). IV. The WPD loop closes for the conserved active site Phenylalanine (Phe182) to make stacking interactions with the substrate. Conformation of the substrate is optimized to aid catalysis. This orientation is perpendicular to that noted in step II. (based on the PTP1B-phosphotyrosine complex). V. Optimized orientation of the substrate ensures catalysis. The cysteinyl-phosphate intermediate as seen at the active of PTP1B. VI. The cysteinyl–phosphate intermediate is hydrolyzed by addition of water by Glutamines of the Q loop. The active site cysteine reverts to its free, de-protonated state with three waters at the active site (based on the PTP1B closed form structure).