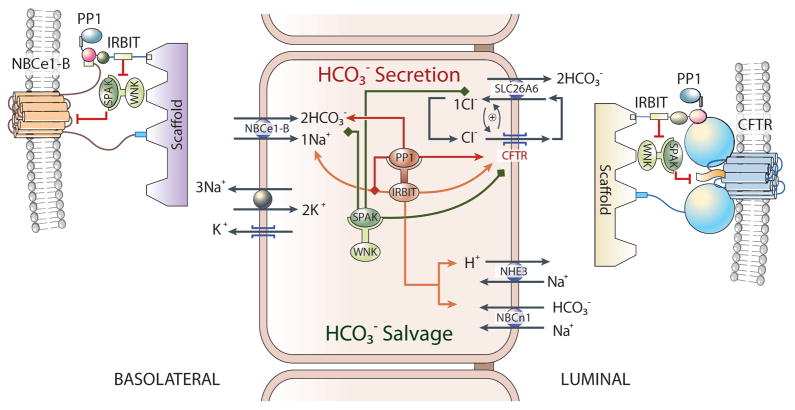
Fig. 5. The IRBIT/PP1 and WNK/SPAK pathways in ductal function.
IRBIT is a key regulator of ductal fluid and HCO3− secretion that regulates both the resting and stimulated states of ductal secretion. PZD scaffolds assemble a basolateral membrane complex composed of NBCe1-B, the WNK/SPAK kinases and IRBIT (which can recruit the phosphatase PP1 to the complex). Similar complex exists in the luminal membrane with CFTR and perhaps Slc26a6 as the major transporters. In the resting state the WNK/SPAK kinases phosphorylate all transporters to reduce their surface expression and thus activity. Part of IRBIT is sequestered by IP3Rs and part is bound to NHE3 and NBCn1-A to activate them and thus affect HCO3− salvage. Upon cell stimulation IRBIT recruits PP1 to the complexes, which overrides the function of the WNK/SPAK pathway and dephosphorylates the HCO3− secreting transporters to increase their surface expression. IRBIT then binds and neutralizes the effect of the HCO3− secreting transporters inhibitory domains. The combined effects stabilize the secretory state of the duct. The mutual stimulation of CFTR and Slc26a6 in the complex further augments ductal secretion.