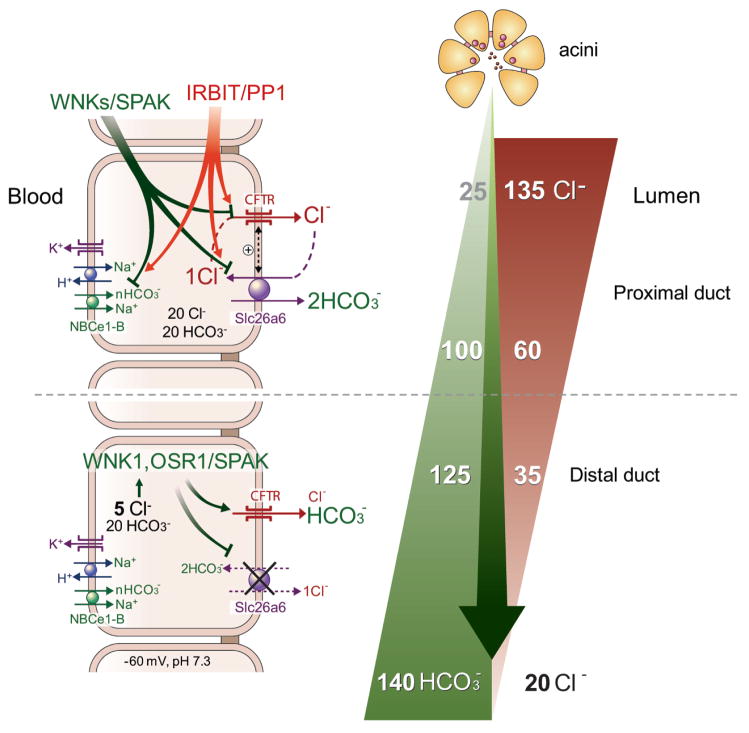
Fig. 6. A model of pancreatic duct fluid and HCO3− secretion.
Ductal fluid and HCO3− secretion is a two stage process. In the proximal duct IRBIT antagonizes the effect of the WNK/SPAK pathway to stimulate ductal secretion. HCO3− accumulates in the duct cytosol by NBCe1-B and exits into the lumen mostly by Slc26a6, which mediates 1Cl−/2HCO3− exchange, with CFTR recycling the Cl−. This result with osmotic secretion of HCO3− and together with transcellular Na+ fluxes through the paracellular pathway drives fluid secretion. The water is secreted by AQP1. The proximal duct thus absorbs part of the Cl− and secretes as much as 100 mM HCO3− to secret large fraction of the fluid in the pancreatic juice. As the fluid arrives the more distal portions of the duct, the reduced luminal Cl− and activated CFTR results in intracellular Cl− concentration ([Cl−]i) of less than 10 mM. The low [Cl−]i activates WNK1 that phosphorylates SPAK/OSP1, which, in turn, acts on CFTR to change its Cl−/HCO3− selectivity, converting it primarily a HCO3− channel. At the same time the WNK/SPAK pathway inhibits the function of Slc26a6 to prevent HCO3− re-absorption. HCO3− efflux by CFTR thus determines the final HCO3− concentration in the secreted fluid.