Abstract
Background
Prognosis conversations are complex phenomena of substantial importance to palliative care (PC), yet these remain poorly understood. This study empirically identifies and describes major types of prognosis conversations that occur in the natural setting of PC consultation.
Methods
We audio-recorded and coded 71 inpatient “goals of care” PC consultations at a large academic medical center in the northeastern United States. We used quantitative Latent Class Analyses for identifying discrete prognosis conversation types and qualitative Dimensional Analyses for more fully describing the process and content of the latent classes.
Results and Conclusions
We observed three discrete types of prognosis conversations, each placing different communication demands upon all participants for achieving goal-concordant care: Navigating Options & Goals (56% of consultations), Facilitating New Goals (23%) and Preparing for End-of-Life (21%). This study provides the first step for developing educational and clinical prognosis communication interventions that are tailored to common decision-making contexts facing seriously ill patients, their families, and PC clinicians.
Introduction
The most common reason for palliative care (PC) consultation is to help seriously ill patients and their families with treatment decision making, often amid frightening and confusing clinical situations. Direct observation of such PC decision-making consultations1 finds that patients, families and PC clinicians frequently engage in conversation about prognosis—resulting in better patient understanding of life expectancy and anticipated treatment effects.2 Importantly, the multicenter Coping with Cancer Study observes that patients who have a better understanding of their prognosis are more likely to receive medical treatments that are aligned with their personal values and goals.3
Clinical prognosis conversations are multidimensional, relational, and dynamic. Each conversation is ultimately unique—formed by the specific clinical situation, by cultural contexts and existing beliefs, and by the communication skills of the participants. Oftentimes, patterns among key features of such complex phenomena can reveal discrete and meaningful groupings (“types,” “classes”) that provide insights about their function within their environment. State-of-the-art concepts of clinical communication endorse this “ecological” approach4–6 to develop more effective education and communication interventions that support patient-centered care. This study takes the first important step along this pathway by identifying and describing the types of prognosis conversations that occur in the natural setting of PC consultation.
To group prognosis conversations into types, we use a statistical modeling approach called Latent Class Analysis (LCA). LCA is specifically designed to identify patterns among multiple components of highly complex phenomena. For this study, we use LCA to identify discrete groups of prognosis conversations based upon patterns among multiple communication elements that we measured for each conversation. After conversations types were empirically grouped by LCA, we then used a qualitative approach called Dimensional Analysis to describe each type more fully. Dimensional Analysis is specifically designed to explore “what all is going on here?” for complex phenomena.
Methods
Overview
We audio-recorded 71 initial PC inpatient consultations to describe the characteristics and determinants of prognosis communication in the natural setting. In addition to audio-recording, we briefly interviewed the PC team and extracted clinical data from the medical record. Sixty-six of these conversations contained some discussion of prognosis.
As described in detail below, we used quantitative methods to empirically classify prognosis conversations into types and then used qualitative methods to describe how each type of conversation differed from the others.
Context, population, and eligibility
This study took place at a 750-bed academic medical center in the northeast United States with a mature inpatient PC consultation service (∼1000 patients/year). Two multidisciplinary PC consult teams are available during weekdays. During the 4-month study period, 12 attending physicians, two nurse practioners, and two PC fellows were present and all were eligible to participate. All English-speaking patients who were at least 21 years of age (or surrogates if decisional capacity was impaired) and referred for “goals-of-care” or “decision making” were eligible to participate. Patients who were enrolled in hospice or who had a Comfort Measures Only plan of care indicated on their Medical Orders for Life-Sustaining Treatments (MOLST) form at the time of consultation were excluded.
Data sources
Recorded consultations
With prior informed consent, we placed digital recorders in unobtrusive locations in the hospital rooms before the PC team entered and retrieved them at the end of the visit. If the clinicians stepped out of the room during the consultation, we later deleted sections of the recording where the clinicians were absent. Our digital recording hardware and method yielded high-fidelity recordings that allow the coder to hear even weak voices amid clinical background noises, such as high-flow oxygen, intravenous pumps and heart rate/respiratory rate monitors.
Medical record
We extracted the following from the standardized PC consultation form: patient age, gender, primary diagnoses, referral reason, referring team/hospital floor, Palliative Performance Scale (PPS) score and Edmonton Symptom Assessment Scale score. For nine participants, the PPS was not completed on the consultation form; however, the medical record provided sufficient information to accurately categorize the PPS into Low (PPS score ≤30), Moderate (40–50) and High (≥60) categories. We collected the following from the medical record and hospital administrative data: race, insurance type, hospital admit date, consult date, and advance directives.
Quantitative Component (Latent Class Analysis)
LCA7 is an iterative statistical modeling method for identifying discrete patterns among the multiple features of complex phenomena, such as conversations. LCA involves four general steps: 1) measurement of conceptually important features that will be used to identify types; 2) identifying the minimum set of features required to define types; 3) defining the number of types best supported by the modeled data; and 4) describing the pattern of features observed within each type.
Conceptual communication features for defining latent classes
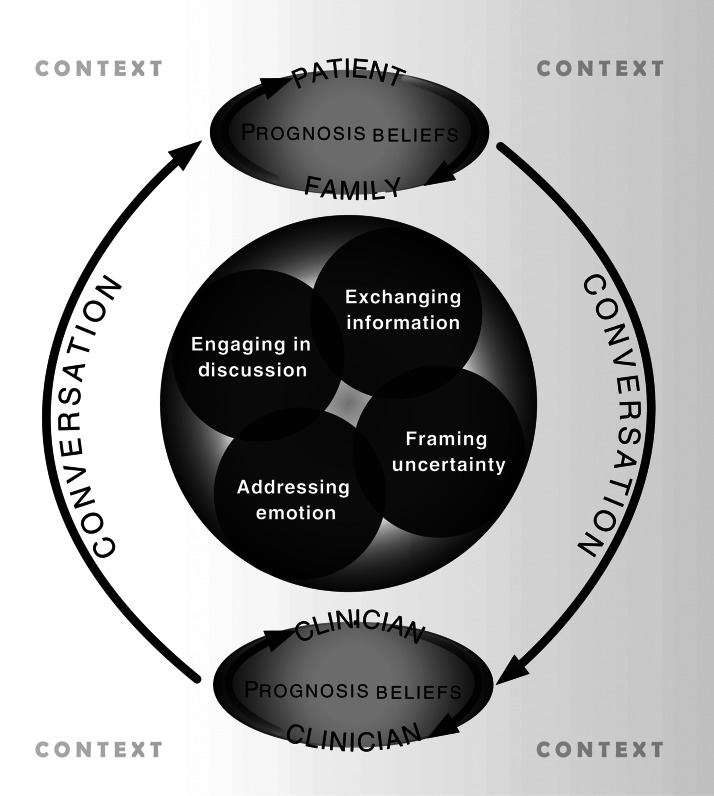
We measured communication behaviors within the following domains defined by the Ecological Model of Prognosis Communication (Fig. 1).6
FIG. 1.
Ecological Model of Prognosis Communication.
Engaging in prognosis discussion
We defined the onset of the prognosis discussion when a request for prognosis information was made by the patient or family, an offer to discuss prognosis was made by the PC team, or a prognosis statement was made by any participant. We identified who initiated the prognosis discussion, when it began within the consultation, and how many conversation segments included prognosis-related talk (requests, offers, information).
Exchanging prognostic information
As described elsewhere,1 we measured the frequency of conversation segments containing a prognosis, the balance of focus between quality of life (QOL) and length of life, the balance in prognosis segments spoken by the PC team compared with the patient/family, and whether prognosis was conditional on pending treatment choices.
Addressing prognosis emotions
Using an established approach,8–10 we coded the frequency and types of negative emotions (e.g., anger, sadness, anxiety/fear) expressed by patients/families throughout the consultation and the types of responses to these emotions by the PC clinician (e.g., empathy, empathy and information, nonempathic). For this analysis of prognosis conversations, we include only those negative emotions that identifiably pertained to prognosis by their proximity to the receipt of prognosis information or by specific mention of prognosis.
Framing prognostic uncertainty
We identified the frequency of prognosis segments that included pessimistic cues (“Unfortunately, I expect that …”) and/or optimistic cues (“The good news is that…”). We also identified the frequency of prognoses that were framed at the population level (e,g., “Thirty percent of people with cancer…”) versus the patient level (e.g., “You have a 30% chance of…”).
Measurement of conversation characteristics
We coded each speaker turn in the conversation, referred to as conversation segments, for the presence of the predefined communication elements (described below). This method has been used in multiple studies of physician-patient communication,11–17 including our prior work.1
We used a detailed manual to train coders for approximately 30 hours over a 2-week period after all data collection was completed. We doubly coded 20% of conversations and used Cohen's kappa to calculate inter-rater reliability (IRR) following Landis and Koch's classification.18 Among the doubly coded sample, coding disagreements were resolved by analysis team consensus. The following demonstrated “strong agreement” (kappa 0.6–0.8) to “near perfect agreement” (kappa 0.8–1.0): prognosis statement, topic of prognosis (QOL versus length of life), population versus individual frame, optimistic and pessimistic cues, expression of negative emotions, and responses to expressed emotions. Whether the prognosis was conditional on a pending treatment choice was coded with “moderate agreement” (kappa 0.47).
Minimum set of features required for defining latent classes
As required for LCA,7,19 we categorized candidate communication behaviors based on their median values, clinically meaningful thresholds, or inflection points in the observed frequency distribution. Based upon exploratory factor analyses of 18 potential candidate variables, we found that eight of these variables exhibited independence of one another within the latent classes and were sufficient for demarcating the boundaries of each class. Therefore, the following represent the final set used for latent class analyses:
1) Initiator of Prognosis Discussion (patient/family initiated in 35% of conversations);
2) Timing of Prognosis Discussion (onset within first 5 minutes of consultation, 61%);
3) Quantity (more than five conversation segments with prognosis discussion, 47%);
4) Topic (exclusively QOL-focused prognosis, 39%);
5) Conditionality (≥1 prognosis conditional on a pending treatment choice, 68%);
6) Prognosis Emotion (≥1 negative prognosis emotion expressed by patient/family, 38%);
7) Optimistic Cues about Prognosis (≥1 optimistic cue, 50%);
8) Pessimistic Cues about Prognosis (≥1 pessimistic cue, 67%).
Number of latent classes
Determining the number of latent classes supported by the data requires judgment. These judgments are based primarily upon consideration of three factors: the proportions of conversations assigned to each of the potential latent classes (i.e., avoiding trivial class sizes), clinical interpretation of the elements that define distinct latent classes, and statistical tests of model fit. Using PROC LCA19,20 in SAS version 9.3 (SAS Institute Inc., Cary, NC), we modeled the final set of eight conversation descriptors for one to six possible latent classes. We found that the data best supported three latent classes for the following main reasons: 1) the class sizes were not trivial (i.e., each type included more than 10 conversations); 2) component features exhibited strong boundaries between types; and 3) model fit statistics (i.e., Akaike information criterion, Bayesian information criterion) demonstrated substantially greater “loss of information” when the model was fit for four or more types. Judgments in the final number of latent classes best supported by the data were made in consultation with the full study team.
Patterns of features within each latent class
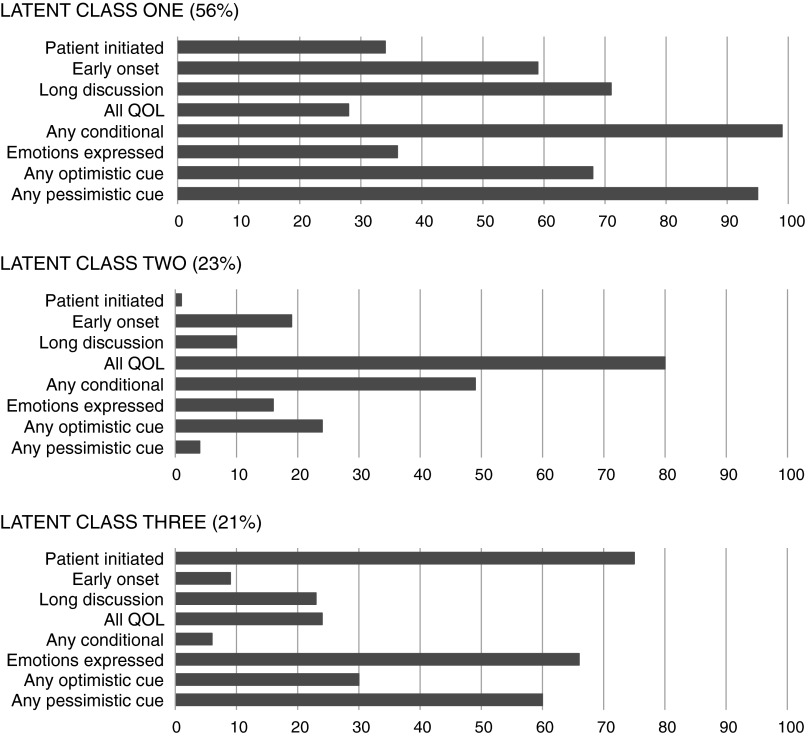
For each latent class, we calculated the proportion of conversations exhibiting each of the eight individual communication features (e.g., “prognosis discussion initiated by the patient/family,” etc.). We display the distribution of communication features for each of the three latent classes in Figure 2.
FIG. 2.
Three latent classes of prognosis conversation.
Distribution of latent classes
We described the distribution of the three latent classes of conversations across the following clinical and demographic factors: patient age, gender, race, insurance status, mean household income by ZIP code, primary life-limiting disease, physical and emotional symptoms (Edmonton Symptom Assessment Scale score), Palliative Performance Scale score, duration of hospitalization preceding consultation, and the degree of family/surrogate participation in the conversation.
Clustering
We evaluated clustering of latent class assignment among the 66 conversations by the 12 PC attending physicians using the following estimate for the intra-class correlation (ICC), as recently defined for multinomial logistic regression models21:
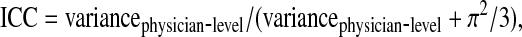 |
where 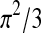 is the residual variance for the logistic model.
is the residual variance for the logistic model.
Qualitative Component (Dimensional Analysis)
We transcribed conversations verbatim, entered them into ATLAS.ti (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) and assigned each conversation to one of three groups (A, B, or C) as defined by quantitative LCA. Two investigators (SN and MM) who did not take part in the LCA used Dimensional Analysis22 to examine each prognosis segment within and across each of the three assigned conversation groups.23 This involved line-by-line coding to identify processes, implicit actions, and meanings for each segment of prognosis communication to identify provisional themes. Two additional qualitative analysts (TQ and SA) subsequently assisted with review and refinement of the provisional themes. Upon completion of all analyses, the full study team considered the quantitative and qualitative descriptions of each conversation type. The group confirmed that the patterns in communication features identified by the LCA (see Fig. 2) were very consistent with the qualitative interpretations of the conversation types.
Human subjects
All clinician and patient participants completed written informed consent and the University of Rochester Research Subjects Review Board approved this study.
Results
Four hundred thirty-eight adult patients were referred for PC consultation during the 4-month recruitment period. Three hundred six of those consultations were requested to help with either “goals of care” or “decision making.” Due to co-occurring consultations and a single study recruiter, we approached 100 unselected patients. Seventy-eight consented to participate. Among consented participants, we missed recording three consultations because they occurred either at night or simultaneously with another participant's consultation. Four recorded consultations did not include sufficient conversation for analyses and five conversations contained no prognosis content. The final sample included 66 patient participants (Table 1). An attending PC physician participated in all conversations. Six of 10 conversations included at least one additional PC clinician and one-quarter included all three (NP, fellow, and attending physician). The patient participated in 58% of conversations; just over half of these also included family members. The remainder involved the PC team and families only.
Table 1.
Description of Sample Characteristics and Distribution by Latent Class
| |
Full sample |
Latent class |
|||
|---|---|---|---|---|---|
| |
N |
Total |
One |
Two |
Three |
| Characteristic | % of conversations | ||||
| All | 66 | 100 | 57 | 23 | 20 |
| Age | |||||
| <60 years | 21 | 32 | 52 | 29 | 19 |
| 60 to <80 years | 24 | 36 | 46 | 25 | 29 |
| ≥80 years | 21 | 32 | 76 | 14 | 10 |
| Gender | |||||
| Women | 30 | 45 | 53 | 30 | 17 |
| Men | 36 | 55 | 61 | 17 | 22 |
| Race | |||||
| Black/AA | 5 | 8 | 60 | 40 | 0 |
| Non-Black/AA | 61 | 92 | 57 | 21 | 21 |
| Insurance status | |||||
| Private | 15 | 23 | 47 | 40 | 13 |
| Medicare | 42 | 63 | 62 | 14 | 24 |
| Medicaid | 9 | 14 | 56 | 33 | 11 |
| Median household income (by ZIP code) | |||||
| ≤$42,100 | 22 | 33 | 68 | 18 | 14 |
| >$42,100 to $54,300 | 23 | 35 | 57 | 17 | 26 |
| >$54,300 to $87,200 | 21 | 32 | 48 | 33 | 19 |
| Marital status | |||||
| Married | 30 | 45 | 63 | 20 | 17 |
| Not married | 36 | 55 | 53 | 25 | 22 |
| Length of stay (pre-consult) | |||||
| ≤2 days | 25 | 38 | 52 | 28 | 20 |
| 3–7 days | 19 | 29 | 53 | 16 | 32 |
| >7 days | 22 | 33 | 68 | 23 | 9 |
| Main diagnosis | |||||
| Cancer | 31 | 47 | 52 | 29 | 19 |
| CHF/COPD | 9 | 14 | 56 | 11 | 33 |
| Stroke | 6 | 9 | 83 | 17 | 0 |
| Other | 20 | 30 | 60 | 20 | 20 |
| Palliative Performance Scale score | |||||
| ≤30 | 28 | 42 | 61 | 25 | 14 |
| 40–50 | 23 | 35 | 47 | 27 | 27 |
| ≥60 | 15 | 23 | 81 | 13 | 6 |
| Severe nausea, pain, or dyspnea? | |||||
| Present | 22 | 33 | 45 | 32 | 23 |
| Absent | 44 | 67 | 64 | 18 | 18 |
| Severe depression or anxiety? | |||||
| Present | 12 | 18 | 67 | 8 | 25 |
| Absent | 54 | 82 | 56 | 26 | 18 |
p values for distribution of all estimates were >0.05.
AA, African American; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
We observed three latent classes of prognosis conversations. Figure 2 shows the distribution of communication characteristics defining each latent class. LCA demonstrated very favorable entropy (0.89), meaning that the vast majority of conversations were definitively categorized to one of the three unique latent classes.7,19 We observed only modest clustering of the three latent classes (ICC=0.17) by the attending PC physician.
Latent Class One (LC-1)
LC-1 accounted for 57% of the conversations and contained the most prognosis information of the three classes. LC-1 conversations nearly always involved discussion of a prognosis that was conditional on pending treatment decisions and frequently contained both pessimistic and optimistic cues. Qualitative analyses revealed LC-1 conversations typically represented situations where participants struggled to understand treatment-specific and overall prognosis for the purposes of matching global treatment goals to the patient's personal values. Sometimes, clinicians' summary characterization of treatment-conditional prognosis (see Appendix, Quote 1) allowed patients to express their personal values in relation to prognosis at the time of initial consultation (Appendix, Quote 2). Other times, participants recognized that disease-oriented treatments were on a poor prognostic trajectory, yet still retained some hope about the short-term success of those treatments. These conversations frequently resulted in plans for time-limited trials with close attention to sources of suffering (Appendix, Quotes 3 and 4).
Latent Class Two (LC-2)
LC-2 accounted for 23% of the conversations, and these conversations were nearly always initiated by the PC team. The discussion of prognosis was typically quite brief and focused exclusively on QOL (i.e., not survival prognosis). Qualitatively, LC-2 conversations involved patients/families who desired greater clinical attention to improving QOL. Prognoses generally took the form of anticipatory guidance regarding “what to expect” from suggested symptom-directed treatments (Appendix, Quotes 5 and 6). When decision making about invasive therapies occurred in LC-2 conversations—which was relatively rare—discussions focused on how the treatment (e.g., feeding tube) would affect QOL goals. (Appendix, Quote 7).
Latent Class Three (LC-3)
LC-3 represented 21% of the conversations, which involved brief discussions of prognosis that were usually initiated by patients/families and almost always absolute in nature (i.e., not conditional on a treatment option). Patient/family frequently expressed negative emotions about prognosis (e.g., sadness, fear, anger) during LC-3 conversations and prognosis was frequently cued with pessimism. Qualitatively, LC-3 involved very few treatment decisions—clinicians were providing anticipatory guidance about life expectancy. Prognoses addressed patient/family requests for more information (Appendix, Quotes 8 and 9), requests to confirm existing suspicions (Appendix, Quote 10), and to identify how to know when death is near (Appendix, Quote 11). A small group of LC-3 conversations appeared to differ from the rest, suggesting a possible subtype. These were initiated by the PC team and represented situations where the clinician was introducing a very poor prognosis to a patient/family whose hopes for improvement appeared discordantly optimistic from those of the clinicians (Appendix, Quotes 12 and 13).
We did not observe substantial nor statistically significant associations between latent class and descriptors of patient demographics, disease type, or clinical condition shown in Table 1.
Discussion
Using an ecological and mixed-methods approach, this study takes the first important steps toward understanding the complex, dynamic, and multidimensional nature of prognosis conversations in PC. LCA revealed three discrete types of prognosis conversations, each having a different set of demands upon their participants.
LC-1, which we refer to as Navigating Options & Goals, is the most common type of prognosis conversation, occurring in more than half of the observed consultations. These situations involve participants who are grappling with treatment options in the setting of diminishing clinical expectations for cure and increasing burdens of therapies. Participants share prognosis information in ways that engage both cognition and emotion, a strategy that promotes better deliberative and intuitive understanding of evidence.24,25 Participants require access to conditional prognosis information for many therapeutic options as well as the skills and supports for maintaining a strong therapeutic alliance during times of heightened emotion, confusion, and potentially conflicting perspectives. One strategy that appears well suited for these types of conversations is the integration of prognosis communication and time-limited trials.26 A time-limited trial is a form of provisional decision making where all participants (clinicians, patient, and/or family) agree to revisit a treatment decision after a period of close observation.27 Time-limited trials allow participants to unite and closely examine the effect(s) of one or more treatments over a short period of time with an explicit intention to use this information for decision making. The observation period offers two key opportunities to patients, families, and clinicians. First is a defined window in time for patients/families to have the opportunity for clarifying the specific outcomes (upon which to prognosticate) of most meaning to the patient given his or her clinical status and personal values. Second, observing the patient's clinical course for a predefined period of time offers additional prognostic information in situations where patients, families, or clinicians perceive initial prognosis estimates are too uncertain for satisfactory decision making.
We describe LC-2 conversations as Facilitating QOL Goals. By and large, participants in these types of prognosis conversations have already identified important QOL goals at the outset of the consultation. This changes the prognostication challenges subtly from LC-1 conversations in two important ways. First, prognosis communication often becomes more tightly focused on prevention or amelioration of specific symptoms, sometimes with potential QOL trade-offs that can require detailed explanation. Second, LC-2 conversations often require the PC clinician to assume a more “advising” than “informing” role about therapies that should enhance QOL. Thus, prognosis communication can represent more explanation for a suggested course of action rather than a means for exploring potential courses of action. This role of expert advisor can be comforting and appropriate for those who are asking for such guidance, but may be less helpful in more deliberative contexts. LC-2 conversations highlight the demand for PC clinicians to be ready to navigate the spectrum of shared decision making in the process of communicating about prognosis.
The third latent class can be described as Preparing for End-of-Life prognosis conversations. There appears to be some important heterogeneity among this latent class of conversations. One subtype involves situations where patients/families are acknowledging that death is near and are asking about “what to expect.” These types of conversations can require PC clinicians to provide sometimes quite detailed descriptions of the expected clinical course with both compassion and presence. Other types of LC-3 conversations generally involve some degree of discordance between the patient's/family's optimistic perceptions about prognosis (including miracles) and the clinicians growing concerns about lack of preparation for an approaching death. These types of conversations can place demands on PC clinicians to communicate about prognosis with humility, to acknowledge the role of conflicting belief systems, and to promote therapeutic partnership amid prognostic disagreement. Some strategies, such as “hoping for the best, and preparing for the worst”28 might provide useful for facilitating a decision-making group to consider starkly opposing possibilities.
This study has important limitations. First and foremost, we sampled from one large academic medical center in the Northeast with a mature PC consultation service. It is likely that the cultural dynamics of other geographic and institutional settings will influence how PC clinicians, patients, and families discuss prognosis. Whether these differences would result in qualitatively different types of prognosis conversations is uncertain. Further typology development will require broader sampling of participants and settings. It is also likely that our conceptual model of prognosis communication is incomplete. Although communicating about prognosis has a long history, quite little is empirically known about the processes that facilitate patient-centered care and decision making. Our model is consistent with current state-of-the-art conceptual models of patient-centered communication,5 but we are likely to learn far more in the coming years.
This is the first study that seeks to define prognosis conversations ecologically—appreciating that the function, adaptations and stressors are not uniform across all prognosis conversations. Our findings highlight the clinical importance of two fundamental PC skills. The first is an acute sense of situational awareness regarding the types of decision-making conversations that unfold during PC consultation. The degree of observation and mindfulness that is required can be challenging to sustain in the busy clinical environment; our findings stress the clinical need to maintain these skills. Second, clinicians will need to develop their flexibility with prognosis communication to adapt to the types of decision-making environments. This study empirically identifies major types” of prognosis conversations and, thus, provides the first step for developing educational and clinical communication interventions that are tailored to the decision-making contexts facing seriously ill patients, their families, and PC clinicians.
Appendix.
Example Quotes Used in Dimensional Analysis
| Quote | Latent class (LC) |
|---|---|
| Navigating Options & Goals(LC-1) | |
| 1 | (Clinician) “Well, she [oncologist] could offer more chemotherapy, the benefits from it sounded like they were pretty low and the burden of it, which some people call the risks but I call the burdens, seemed fairly high in that there could be complications and infection and things that might actually make you feel worse instead of better.” |
| 2 | (Patient) “I don't really want to try anymore chemo or anything if it's going [to], you know, make me live longer but miserably.” |
| 3 | (Clinician) “I think it's probably going to take us a few days to figure that out. And some of it's just seeing how he does, and if it is worth giving him the treatments to do that. And all of these treatments are fairly easy treatments…” |
| (Family) “Right.” | |
| (Clinician) “…so they're not particularly invasive.” | |
| (Family) “Right, but it seems that he's digressing [sic] instead of improving.” | |
| (Clinician) “It may be that he stays that way and then we just say keeping him comfortable is the best we can do. And we're okay with that, if that's what you think you would want.” | |
| 4 | (Clinician) “[If] your ideas of what you guys would like is the same [it] would be to give another two units of blood if she needed it. And if things continued to bleed or not improve with the conservative therapy of the medications that we're giving, at that point then we would make a shift to a more comfort-like plan of care.” |
| Facilitating QOL Goals (LC-2) | |
| 5 | (Clinician) “[T]here are some medications to help you with this shortness of breath but they might add a little bit to the sleepiness.” |
| 6 | (Clinician) “When you first start you may notice it makes you a little bit sleepy. But that gets better the longer you take it.” |
| 7 | (Clinician) “They're talking about putting a tube in your stomach to feed you…” |
| (Patient) “Yeah.” | |
| (Clinician) “…now, that won't stop this from happening, you're still gonna bring up mucous and such.” | |
| (Patient) “Oh, they haven't told me that.” | |
| (Clinician) “Yeah, it won't stop the mucous issue. I mean it will give you some nutrition but, it won't stop the mucous from happening cause you still make spit.” | |
| (Patient) “Yeah.” | |
| (Clinician) “It would take away the need to eat from above and you would get some nutrition but it won't, it won't stop the choking and it won't take away the progression of the cancer.” | |
| (Patient) “Oh, oh, I don't know.” | |
| Preparing for End of Life (LC-3) | |
| 8 | (Clinician) “I think that you probably have the 3-month or less sort of window there. You know there's…both ends of that but something could happen tonight [or] you could be sitting here 6 months from now. You have 2 weeks or 3 months, hard for me to say. Um, you know your breathing looks pretty easy, you don't look like you're going to leave us in the next week or two, but again that could change in a minute. I think that's sort of the number you're looking at.” |
| 9 | (PC clinician) “What is your understanding? And I'm going to start there a little bit so I can help you.” |
| (Patient) “Maybe 4, 6 months.” | |
| (PC clinician) “Is that your understanding from the doctors that talked to you last?” | |
| (Patient) “I guess for awhile, yeah.” | |
| (PC clinician) “My sense is that you're right. We're talking kind of in a realm of many weeks to a few months. It's kind of that spectrum of time. We're not looking at days and we're not looking at years.” | |
| 10 | (Patient) “I mean that the end of my life is coming, probably within a matter of weeks, particularly if, uh, the kidneys blow out because I refuse to go beyond this and, um.” |
| (PC clinician) “You mean with dialysis?” | |
| (Patient) “Exactly.” | |
| 11 | (Clinician) “We're going to expect people who are relatively bedbound to have some things; what often comes is a urinary infection, pneumonia, a bedsore, an infection. And that often is, is, sort of the beginning of the end-of-life course.” |
| 12 | (Clinician) “So as much as we'd like to be able to do things, ’cause that's what we do as doctors, to prolong his life. Other than the antibiotics that we can give him now there's not much that you can do. And based upon how he's looking, he probably only has weeks, at most months, to live…[later in same conversation] We don't think there's anything that will really prolong his life with the disease…that he has. It's a very difficult disease.” |
| 13 | (Clinician) “With the damage done that's, it's going to be hard to undo. I don't think it's going to be able to be undone to tell you the truth…Yeah, I wish it [could be different] but I don't see it happening…But, I do think we can make you feel better.” |
Acknowledgments
This work was funded by research grants from the National Palliative Care Research Center and the Greenwall Foundation. Dr. Gramling was supported by the National Palliative Care Research Center. Dr. Alexander was supported by the Department of Veterans Affairs (RCD 07-006). Dr. Metzger was supported by the National Institute for Nursing Research (F31NR012084). We thank Vi Luong for her graphics expertise and support. We thank the University of Rochester Medical Center Inpatient Palliative Care clinicians, patients, and families for their dedication to research that will enhance care for people with serious illness.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gramling R. Norton SA. Ladwig S, et al. Direct observation of prognosis communication in palliative care: a descriptive study. J Pain Symptom Manage. 2013;45:202–212. doi: 10.1016/j.jpainsymman.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Temel JS. Greer JA. Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol. 2011;29:2319–2326. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 3.Mack JW. Weeks JC. Wright AA. Block SD. Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Street RL., Jr Gordon H. Haidet P. Physicians' communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc Sci Med. 2007;65:586–598. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein RM. Street RL. Patient-Centered Communication in Cancer Care. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 6.Gramling R. Carroll T. Epstein R. Prognostication in advanced illness. In: Goldstein N, editor; Morrison S, editor. Evidence-Based Practice of Palliative Medicine. 1st. Elsevier Press; 2013. [Google Scholar]
- 7.Hagenaars JA. McCutcheon AL. Applied Latent Class Analysis. New York: Cambridge University Press; 2002. [Google Scholar]
- 8.Alexander SC. Pollak KI. Morgan PA, et al. How do non-physician clinicians respond to advanced cancer patients' negative expressions of emotions? Support Care Cancer. 2011;19:155–159. doi: 10.1007/s00520-010-0996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak KI. Arnold RM. Jeffreys AS, et al. Oncologist communication about emotion during visits with patients with advanced cancer. J Clin Oncol. 2007;25:5748–5752. doi: 10.1200/JCO.2007.12.4180. [DOI] [PubMed] [Google Scholar]
- 10.Tulsky JA. Arnold RM. Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program: A randomized trial. Ann Intern Med. 2011;155:593–601. doi: 10.1059/0003-4819-155-9-201111010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander S. Keitz S. Sloane R. Tulsky J. A controlled trial of a short course to improve medical house staff communication with patients at the end of life. Acad Med. 2006;81:1008–1012. doi: 10.1097/01.ACM.0000242580.83851.ad. [DOI] [PubMed] [Google Scholar]
- 12.Back AL. Arnold RM. Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167:453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 13.Brown R. Bylund CL. Eddington J. Gueguen JA. Kissane DW. Discussing prognosis in an oncology setting: Initial evaluation of a communication skills training module. Psycho-Oncology. 2010;19:408–414. doi: 10.1002/pon.1580. [DOI] [PubMed] [Google Scholar]
- 14.Bylund CL. Brown RF. di Ciccone BL, et al. Training faculty to facilitate communication skills training: Development and evaluation of a workshop. Patient Educ Couns. 2008;70:430–436. doi: 10.1016/j.pec.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Kennifer S. Alexander S. Pollak K, et al. Negative emotions in cancer care: Do oncologists' responses depend on severity and type of emotion? Patient Educ Couns. 2009;76:51–56. doi: 10.1016/j.pec.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollak K. Arnold R. Jeffreys A. Alexander SC. Olsen M. Abernethy AP. Skinner CS. Rodriguez KL. Tulsky JA. Oncologist communication about emotion during visits with advanced cancer patients. J Clin Oncol. 2007;25:48–52. doi: 10.1200/JCO.2007.12.4180. [DOI] [PubMed] [Google Scholar]
- 17.Robinson T. Alexander S. Hays M, et al. Patient-oncologist communication in advanced cancer: Predictors of patient perception of prognosis. Support Care Cancer. 2008;16:803–811. doi: 10.1007/s00520-007-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis JR. Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 19.Lanza ST. Collins LM. Lemmon DR. Schafer JL. PROC LCA: A SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14:671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PROC LCA & PROC LTA [computer program] State College, PA: Penn State University; 2011. Version 1.2.7. [Google Scholar]
- 21.Rabe-Hesketh S. Skrondal A. Understanding variability in multilevel models for categorical responses. Paper presented at AERA Annual Meeting; Vancouver, Canada. Apr, 2012. [Google Scholar]
- 22.Shatzman L. Dimensional analysis: Notes on an alternative to the grounding of theory in qualitative research. In: Maines DR, editor. Social Organization and Social Process. New York: 1991. pp. 303–314. [Google Scholar]
- 23.Ayres L. Kavanaugh K. Knafl KA. Within-case and across-case approaches to qualitative data analysis. Qual Health Res. 2003;13:871–883. doi: 10.1177/1049732303013006008. [DOI] [PubMed] [Google Scholar]
- 24.Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- 25.Kahneman D. A perspective on judgment and choice: Mapping bounded rationality. Am Psychol. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- 26.Quill TE. Holloway R. Time-limited trials near the end of life. JAMA. 2011;306:1483–1484. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 27.Epstein RM. Gramling RE. What is shared in shared decision making? Complex Decisions when the evidence is unclear. Med Care Res Rev. 2013;70(1 Suppl):94S–112S. doi: 10.1177/1077558712459216. [DOI] [PubMed] [Google Scholar]
- 28.Back AL. Arnold RM. Quill TE. Hope for the best, and prepare for the worst. Ann Intern Med. 2003;138:439–443. doi: 10.7326/0003-4819-138-5-200303040-00028. [DOI] [PubMed] [Google Scholar]