Table 1.
Synthesis of quaternized donor heterocycles.
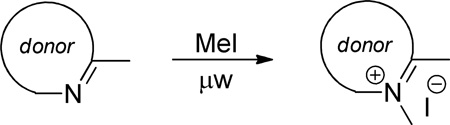 | ||||||
|---|---|---|---|---|---|---|
| heterocycle | donor code |
Brooker basicity a |
product | temp (°C) |
time (min) |
yield (%) |
| indolenine | I | 260 | 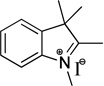 |
120 | 5 | 80 |
| benzoxazole | O | 345 | 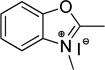 |
100 | 20 | 51 |
| benzothiazole | S | 375 | 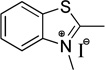 |
120 | 20 | 93 |
| thiadiazole | TD | - | 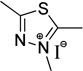 |
90 | 20 | 98 |
| quinoline | Q | 730 | 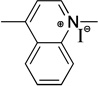 |
r.t.b | 15 | 95 |
From reference 15.
Reaction stirred at room temperature for time indicated without microwave heating.
