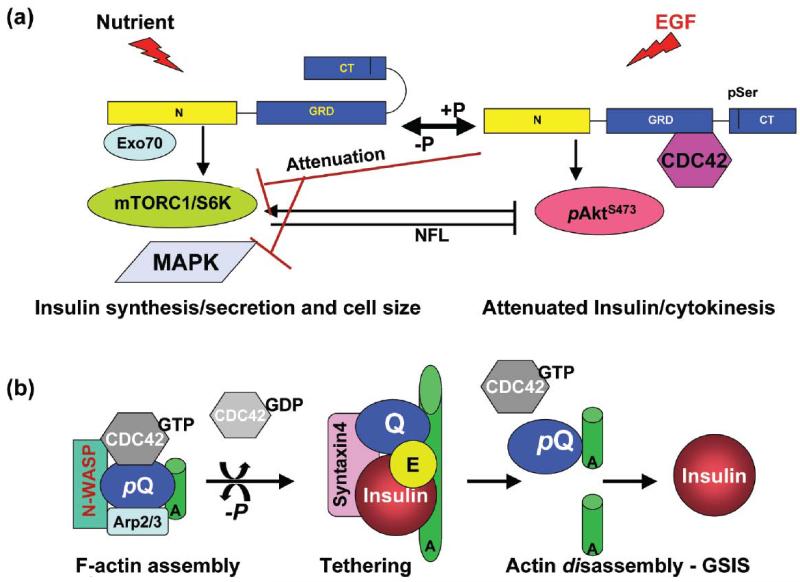
Figure 3.
IQGAP1 as a phospho-serine-sensitive conformational-switch regulating β-cell function. (a) IQGAP1 couples cell size and division. Left, in response to nutrients (glucose), Exocyst-bound IQGAP1-N binds mTORC1 (and Sec61β translocon subunit, not shown) and promotes insulin synthesis and secretion, while auto-inhibition by folding of the C-terminus prevents Cdc42 from binding [53, 77, 78, 79]. Right, in response to EGF (or low nutrients), pIQGAP1S1443 has an open C-terminus, binds active Cdc42, and attenuates insulin synthesis and secretion by attenuating the S6K1T389 activity, and thus attenuating the negative feedback loop (NFL, black arrow) on AktS473 and diverting its activity towards cell division [53, 77, 78, 79]. (b) IQGAP1 known modulation of F-actin dynamics as applied to regulating glucose-stimulated insulin secretion (GSIS). Activated pIQGAP1S1443 (pQ) recruits active Cdc42 (Cdc42-GTP), N-WASP/Arp2/3 complex to assemble F-actin into meshwork [68, 69, 73-75] that help dock the vesicles, but inhibits secretion. Dephosphorylated IQGAP1 (Q) disengages Cdc42-GTP and the actin bundling machinery, and binds the exocyst and the t-SNARE syntaxin 1A, to tether the insulin vesicles/granules to the plasma membrane, culminating in actin disassembly [68, 69, 73-75] and insulin release (GSIS). A new cycle of actin assembly begins with IQGAP1 phosphorylation (pQ). P: phosphorylation by PKCε; E: Exocyst; A: actin filaments or cables.