FIGURE 7:
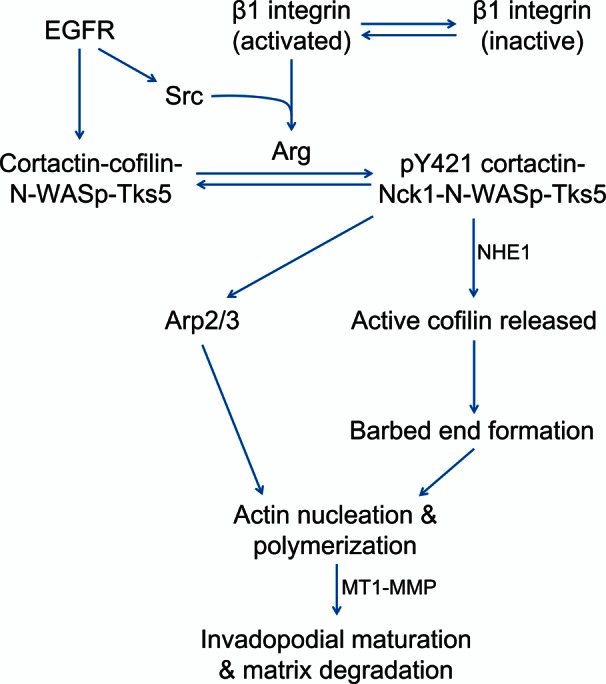
Model of β1 integrin–dependent regulation of invadopodia. β1 integrin is activated in invadopodium precursors, leading to increased local β1 integrin adhesion in the protrusion/core, which is a key switch that drives Arg activation, cortactin phosphorylation, and MMP recruitment during invadopodial maturation. It is proposed that β1 integrin-EGFR cross-talk activates Arg by a three-step mechanism: 1) Arg binding to the β1 integrin cytoplasmic tail is believed to disrupt its autoinhibitory conformation, unmasking the Y272 autophosphorylation site and Y439 on the activation loop. 2) Integrin-mediated clustering of Arg likely facilitates autophosphorylation on Y272. 3) EGFR activation induces Src-dependent Arg phosphorylation on Y439, resulting in full Arg kinase activation. Arg-dependent cortactin phosphorylation results in the recruitment of NHE-1 to increase the local intracellular pH, resulting in disruption of the inhibitory interaction between cortactin and cofilin and recruitment of Nck1 for N‑WASp activation to induce Arp2/3-dependent actin polymerization. In this way, β1 integrin acts as a critical upstream regulator of Arg kinase activity, actin polymerization, and subsequent protease recruitment to promote efficient invadopodial matrix degradation.
