dSkp2 regulates cell cycle progression by antagonizing Dap in Drosophila, which resolves the question of whether dSkp2 has a role in regulating Dap stability and suggests the possibility of using Drosophila as a model system in which to study Skp2-mediated tumorigenesis.
Abstract
Cell cycle progression is controlled by a complex regulatory network consisting of interacting positive and negative factors. In humans, the positive regulator Skp2, an F-box protein, has been a subject of intense investigation in part because of its oncogenic activity. By contrast, the molecular and developmental functions of its Drosophila homologue, dSkp2, are poorly understood. Here we investigate the role of dSkp2 by focusing on its functional relationship with Dacapo (Dap), the Drosophila homologue of the cyclin-dependent kinase inhibitors p21cip1/p27kip1/p57kip2. We show that dSkp2 interacts physically with Dap and has a role in targeting Dap for ubiquitination and proteasome-mediated degradation. We present evidence that dSkp2 regulates cell cycle progression by antagonizing Dap in vivo. dSkp2 knockdown reduces cell density in the wing by prolonging the cell doubling time. In addition, the wing phenotype caused by dSkp2 knockdown resembles that caused by dap overexpression and can be partially suppressed by reducing the gene dose of dap. Our study thus documents a conserved functional relationship between dSkp2 and Dap in their control of cell cycle progression, suggesting the possibility of using Drosophila as a model system to study Skp2-mediated tumorigenesis.
INTRODUCTION
In eukaryotes, cell cycle progression requires the activation of a series of cyclin-dependent protein kinases (CDKs) in combination with their partner cyclins at specific points (Morgan, 1995). For example, progression through the G1 restriction point in animal cells is controlled by the Cdk4/CycD and Cdk6/CycD complexes, and the transition from G1 to S phase is achieved through the Cdk2/CycE complex (Vermeulen et al., 2003). CDK inhibitors (CKIs) are negative regulators of cell cycle progression by inhibiting the activity of CDK–cyclin complexes. Members of the Cip/Kip family of CKIs inhibit the G1 to S transition by associating with, and inhibiting the activity of, the Cdk2/CycE complex (Wojda, 2000).
In humans, there are three Cip/Kip family members: p21cip1, p27kip1, and p57kip2. The activities of these proteins are subject to negative regulation by SCFSkp2 (Yu et al., 1998; Amati and Vlach, 1999; Carrano et al., 1999; Tsvetkov et al., 1999; Kossatz et al., 2004; Nakayama et al., 2004; Pagano, 2004; Bond et al., 2006; Pateras et al., 2006; Yung et al., 2007). SCFSkp2 is a ubiquitin E3 ligase complex, with its Skp2 subunit, an F-box protein, providing substrate specificity through direct protein–protein interactions. Skp2 has been shown to promote the G1 to S transition and proliferation in a mouse model (Zhu, 2010). Skp2−/− animals are viable, but cells from mutant mice contain markedly enlarged nuclei with polyploidy and multiple centrosomes (Zhu, 2010). These cells also show reduced growth rate and increased apoptosis. As an important regulator of cell cycle control, Skp2 overexpression is a characteristic feature of a variety of cancers (Gstaiger et al., 2001; Signoretti et al., 2002; Ben-Izhak et al., 2003; Wang XC et al., 2012; Wang Z et al., 2012).
Cell cycle progression in Drosophila is believed to be controlled by highly conserved cyclins and CDKs (Follette and O'Farrell, 1997). Unlike humans, Drosophila has only one known Cip/Kip family member, Dacapo (Dap). Dap negatively regulates the G1 to S transition by inhibiting the CycE/Cdk2 complex, an action that is mediated by the conserved core CDI domain of Dap (de Nooij et al., 1996; Lane et al., 1996). Despite its critical role in cell cycle control, precisely how the stability of Dap protein is regulated is largely unclear. This is in part because Dap appears to have divergent phosphorylation sites critical for ubiquitination (Adams et al., 1996; Morisaki et al., 1997; Huang et al., 2006). Although it was proposed that CG9772 encodes Drosophila Skp2 (dSkp2; Moberg et al., 2004), the genetic and molecular functions of this gene were poorly studied. In a recent report, Ghorbani et al. (2011) presented genetic evidence that established a role for dSkp2 in maintaining diploidy of mitotic cells during development. However, they did not observe a role of dSkp2 in regulating Dap stability, raising the question of whether these two proteins might indeed exhibit a functional relationship that is conserved in cell cycle regulation. Here we describe genetic and molecular studies that specifically investigate the functional relationship between dSkp2 and Dap. Our results show that dSkp2 plays a role in targeting Dap for degradation and has a developmental function interacting with that of Dap in controlling cell cycle progression.
RESULTS
dSkp2 interacts with Dap and has a role in regulating Dap protein level in Drosophila
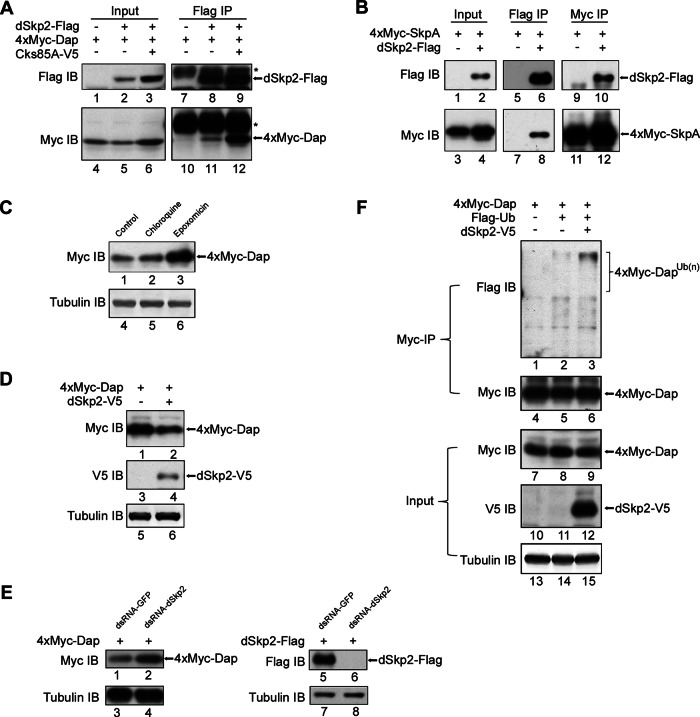
To investigate the question of whether there is a functional relationship between dSkp2 and Dap in Drosophila, we determined specifically whether dSkp2 and Dap can physically interact with each other. We transiently expressed tagged proteins 4xMyc-Dap and dSkp2-Flag in Drosophila S2 cells and performed coimmunoprecipitation (coIP) assays. We used an anti-Flag antibody to precipitate dSkp2 from the cell extracts and an anti-Myc antibody in Western blots to detect the presence of Dap in the precipitated products. Our results show that 4xMyc-Dap was coimmunoprecipitated when, and only when, dSkp2-Flag was coexpressed in S2 cells (Figure 1A, lane 11; dSkp2–Dap interaction was enhanced by Cks85A, lane 12, a result to which we return in the Discussion). These results suggest a physical interaction between dSkp2 and Dap.
FIGURE 1:
dSkp2 interacts with Dap and regulates Dap stability in S2 cells. (A) CoIP between dSkp2 and Dap. S2 cells were transfected with the indicated combination of plasmids. The anti-Flag antibody was used for IP. Antibodies used for immunoblotting (IB) are indicated on the left. Cks85A is the Drosophila homologue of Cks1; its expression in S2 cells increased the amount of coIP products (lane 12; see Discussion). Asterisk, heavy chain of immunoglobulin G. (B) CoIP between dSkp2 and SkpA. S2 cells were transfected with the indicated combination of plasmids. Anti-Flag antibody (lanes 5–8) or anti-Myc antibody (lanes 9–12) were used for IP, followed by IB using anti-Flag and anti-Myc antibodies to detect dSkp2-Flag and 4xMyc-SkpA, respectively. (C) Dap protein level in S2 cells is sensitive to proteasome inhibitor. S2 cells were transfected with the 4xMyc-dap plasmid and then treated with the indicated inhibitors (chloroquine and epoxomicin; see the text) for 5 h before cell harvest. Total amount of 4xMyc-Dap in cells was detected by IB using the anti-Myc antibody (lanes 1–3). Tubulin (lanes 4–6) was blotted as loading control. (D) Dap protein level in S2 cells is sensitive to dSkp2 overexpression. S2 cells were cotransfected with the indicated plasmids and cycloheximide (CHX) was added to block translation 5 h before cell harvest. Total protein was detected in IB using the indicated antibodies. Tubulin (lanes 5 and 6) is loading control. (E) S2 cells were first treated with control (GFP) dsRNA (lanes 1, 3, 5, and 7) or dSkp2 dsRNA (lanes 2, 4, 6, and 8) for two times, each lasting 3 d. Cells were then transfected with plasmids expressing 4xMyc-Dap before harvesting (48 h later) for the detection of the total amount of 4xMyc-Dap (lanes 1 and 2). RNAi efficiency was estimated by the reduction in the amount of dSkp2-Flag upon RNAi treatment (lanes 5 and 6). Tubulin (lanes 3, 4, 7, and 8) represents loading control. (F) dSkp2 overexpression enhances the ubiquitination status of Dap. S2 cells were transiently transfected with the indicated plasmids. Whole-cell extracts were prepared for coIP by the anti-Myc antibody. Anti-Flag antibody was used to detect the ubiquitinated species of 4xMyc-Dap as marked. Input represents 1% of the extracts used in coIP.
The following three sets of experiments performed in S2 cells further suggest that dSkp2 is a component of a conserved SCF E3 ligase complex, SCFdSkp2, that has a role in targeting Dap for ubiquitination and proteasome-mediated degradation. First, our coIP assays using extracts from S2 cells expressing tagged proteins 4xMyc-SkpA and dSkp2-Flag show that, consistent with the recent report by Ghorbani et al. (2011), dSkp2 could physically interact with SkpA, a component of SCF complexes (see Figure 1B and legend for details). Second, the stability of Dap (as Myc-Dap fusion) in S2 cells was sensitive to the proteasome inhibitor epoxomicin (see Figure 1C, lane 3) but not the lysosome inhibitor chloroquine (lane 2; see control in lane 1). Of importance, coexpression of dSkp2 (as dSkp2-V5 fusion) reduced Dap protein levels in S2 cells (Figure 1D), whereas RNA interference (RNAi) against dSkp2 increased Dap protein levels (Figure 1E). These results suggest a role of dSkp2 in regulating Dap stability. Third, we expressed tagged proteins Flag-ubiquitin and 4xMyc-Dap in S2 cells, with or without coexpression of dSkp2-V5. Our results show that coexpression of dSkp2-V5 increased the amount of the polyubiquitinated species of Dap (Figure 1F, lanes 2 and 3), suggesting that dSkp2 has a role in targeting Dap for ubiquitination.
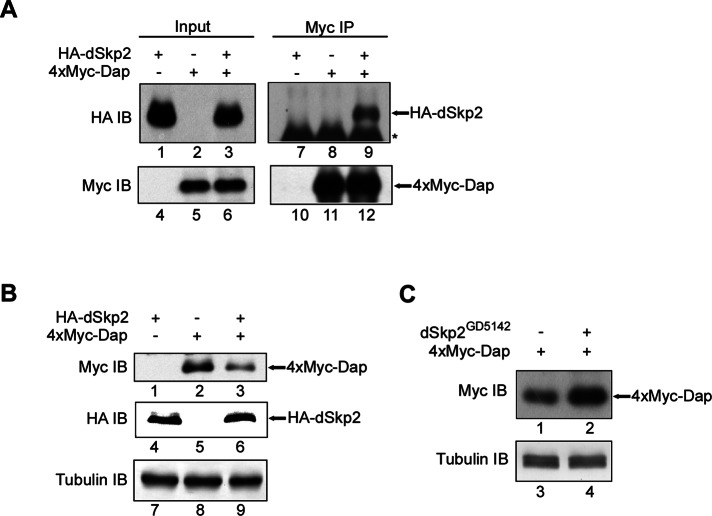
To extend our studies performed in S2 cells and further evaluate the molecular relationship between dSkp2 and Dap, we conducted experiments using Drosophila tissue extracts. Here our goal was to use fly tissues as a host to investigate whether these two proteins can also interact as seen in S2 cells. We generated transgenic flies containing UAS-expression constructs for tagged proteins hemagglutinin (HA)-dSkp2 and 4xMyc-Dap. We expressed these proteins in the eye using the GMR-Gal4 driver, and performed coIP experiments in extracts prepared from the fly heads. We used the anti-Myc antibody to precipitate Dap in head extracts, and used an anti-HA antibody in Western blots to detect the presence of Skp2 in the precipitated products. Our coIP results further confirmed the interaction between dSkp2 and Dap in Drosophila tissues (Figure 2A; see lane 9). To evaluate the role of dSkp2 in regulating Dap stability in host tissues of Drosophila, we determined the amount of 4xMyc-Dap in the extracts from fly heads with or without coexpression of HA-dSkp2. Our Western blotting results show that coexpression of HA-dSkp2 in the eye reduced the amount of 4xMyc-Dap (Figure 2B, lane 3; see lane 2 as control), whereas coexpression of dSkp2 RNAi led to an appreciable increase in the amount of 4xMyc-Dap (Figure 2C, lane 2; see lane 1 as control). These results provide further support to our suggestion that Dap stability is regulated by dSkp2.
FIGURE 2:
dSkp2 interacts with Dap and regulates Dap protein level in Drosophila tissues. (A) CoIP analysis detecting dSkp2-Dap interaction. Extracts were prepared from the adult heads of flies with the indicated genotypes: GMR>HA-dSkp2 (lanes 1, 4, 7, and 10), GMR>4xMyc-dap (lanes 2, 5, 8, and 11), and GMR>HA-dSkp2 + GMR>4xMyc-dap (lanes 3, 6, 9, and 12). The immunoprecipitates were pulled down with the anti-Myc antibody, and the anti-HA antibody was used in IB to detect the presence of HA-dSkp2. (B) dSkp2 regulates Dap stability. Extracts were prepared from the adult heads of flies as in A. Total protein level in the extracts was detected in IB using the indicated antibodies. Tubulin (lanes 7–9) is loading control. (C) dSkp2 knockdown in Drosophila eyes leads to accumulated Dap proteins in tissue extracts. Extracts were prepared from the adult heads of flies with the indicated genotypes: GMR>4xMyc-dap (lanes 1 and 3) and GMR>4xMyc-dap + GMR>dSkp2GD5142 (lanes 2 and 4). Myc-tagged Dap protein level was determined by Western blotting using the Myc antibody. Tubulin (lanes 3 and 4) represents loading control.
dSkp2 plays a role in normal wing and eye patterning in Drosophila
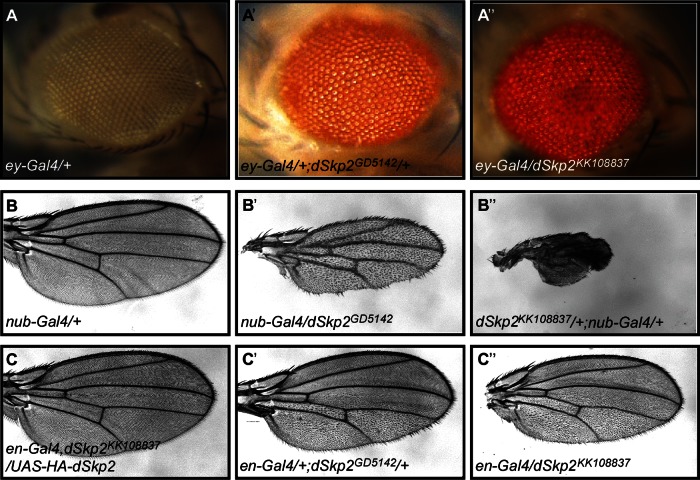
To investigate the biological significance of the association of dSkp2 with Dap, we analyzed a null allele, Skp2ex9, generated in Andrew Swan's lab (Ghorbani et al., 2011). Our results confirmed the reported early pupal lethality in homozygous animals (data not shown), demonstrating that dSkp2 is an essential gene for animal survival. To circumvent the lethality problem and facilitate tissue-specific analyses (see later discussion), we evaluated the effects of ubiquitous dSkp2 knockdown and compared them with those caused by dSkp2 mutation. Our results show that ubiquitous knockdown of dSkp2, using the drivers of daughterless-Gal4 (da-Gal4), tubulin-Gal4 (tub-Gal4), or actin-GAL4 (act-Gal4), led to similar early pupal lethality (data not shown). Of importance, such lethality can be rescued by coexpression of a wt dSkp2 transgene (data not shown), documenting the specificity of the RNAi lines used in this work.
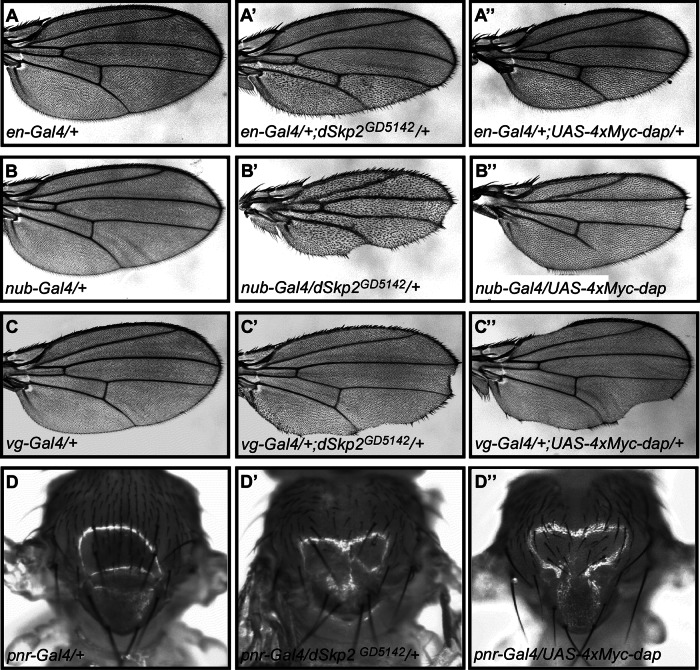
dSkp2 was also identified recently in a systematic phenotypic screening of F-box genes with multiple developmental roles (Dui et al., 2012). Tissue-specific knockdown of dSkp2, using two independent RNAi lines, dSkp2GD5142 and dSkp2KK108837, caused patterning defects in both the eye and the wing (see Supplemental Figure S1 for the RNAi knockdown efficiency and Supplemental Table S1). dSkp2 knockdown by RNAi in either the posterior region of the wing (driven by engrailed-Gal4/en-Gal4; Figure 3, C′ and C′′) or the entire wing (driven by nubbin-Gal4/nub-Gal4; Figure 3, B, B′, and B′′) led to a wing hair spacing phenotype. In particular, the space between wing hairs became larger, with irregular orientation and frequent clustering of wing hairs (see also Supplemental Figure S2). Furthermore, relative to wild-type or untargeted regions, RNAi-expressing regions became smaller (see Figure 3, B′ and B′′, for effects on the entire wing and Figure 3, C′ and C′′, for effects on the posterior region). These phenotypes were completely rescued by coexpressing dSkp2 from the transgenes of either UAS-dSkp2 or UAS-HA-dSkp2 (Figure 3C and data not shown). Two additional independent RNAi lines, dSkp2JF01326 and dSkp2HMS00116, caused similar phenotypes, although with different severity, likely reflecting differences in RNAi strength (Supplemental Table S1). RNAi strength for each of the four lines tested was enhanced by simultaneous overexpression of dicer2, an RNAi-machinery protein (Supplemental Table S1). Taken together, these results suggest a role of dSkp2 in cell proliferation and patterning during Drosophila development. The role of dSkp2 in patterning was also detectable in the eye, where dSkp2 knockdown (driven by eyeless-Gal4/ey-Gal4) caused rough eyes (Figure 3, A, A′, and A′′).
FIGURE 3:
Tissue-specific knockdown of dSkp2 causes developmental defects. (A, A′, and A′′) Rough eyes induced by dSkp2 knockdown in the eye (with the use of the ey-Gal4 driver). Results from two independent RNAi lines are shown. (B′, B′′) nub-Gal4-driven expression of these two dSkp2 RNAi lines in the entire wing results in a similar wing hair spacing phenotype (WHS; see the text for details). Control is shown in B. (C′, C′′) dSkp2 knockdown in the posterior region of the wing (driven by en-Gal4) leads to the WHS phenotype in the posterior region only. This phenotype is fully rescued by a simultaneous overexpression of HA-dSkp2 (C).
dSkp2 regulates cell cycle progression in Drosophila wing discs
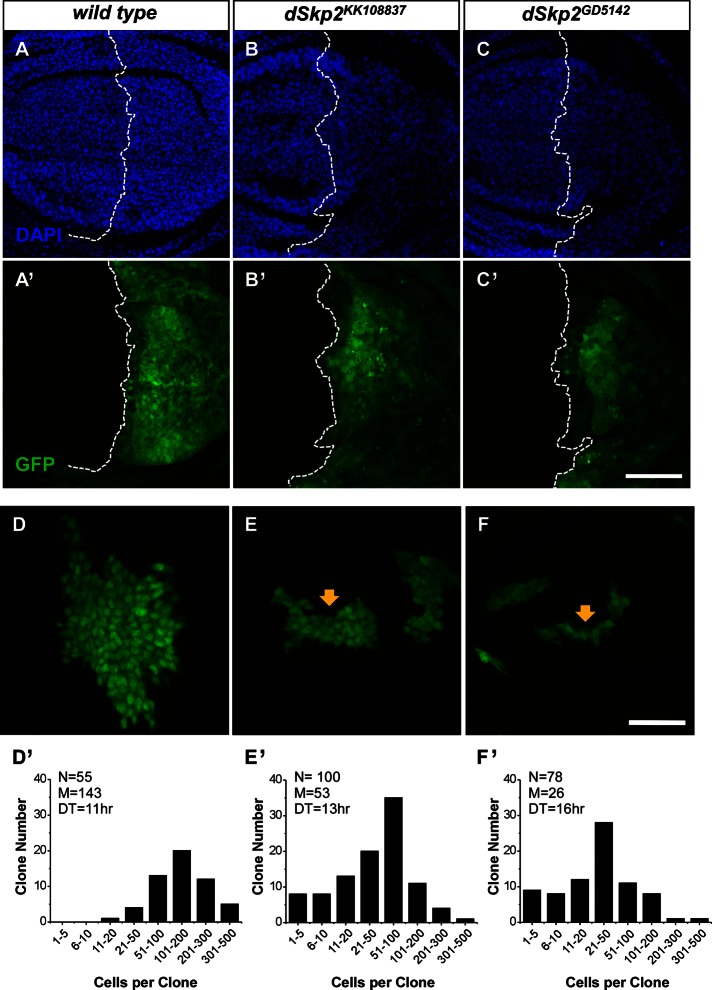
To investigate the underlying defects of the wing hair spacing phenotype caused by dSkp2 knockdown, we determined whether cell number was altered in the adult wing. Supplemental Figure S2 shows that, relative to wild-type wings, dSkp2 RNAi-treated wings had a significantly reduced number of hairs within areas of the same size, indicating a decrease in cell density. Cell density change has generally been attributed to specific cell cycle alterations that uncouple cell growth from cell division (Weigmann et al., 1997; Neufeld et al., 1998; Swan and Schupbach, 2007). To test this possibility, we measured cell density directly in wing discs that had been stained with 4′,6-diamidino-2-phenylindole (DAPI). As shown in Figure 4, in the posterior region of the wing disc with dSkp2 knockdown (driven by en-Gal4), cell density is significantly lower than the wild-type control (Figure 4, B and C).
FIGURE 4:
Knockdown of dSkp2 slows cell proliferation in the wing discs. (A–C) DAPI staining of wing imaginal discs showing nuclear density differences in the posterior region of the disc with (B, C) or without (A) dSkp2 knockdown (driven by en-Gal4 in combination with UAS-dcr2 at 29°C). GFP signals mark the expression domain of en-Gal4 (B′, C′). (A′) The en-Gal4, UAS-GFP control. The genotypes are UAS-dcr2/+; en-Gal4, UAS-GFP/+ (A, A′), UAS-dcr2/+; en-Gal4, UAS-GFP/dSkp2KK108837 (B, B′), and UAS-dcr2/+; en-Gal4, UAS-GFP/+; dSkp2GD5142/+ (C, C′). Scale bar, 50 μm. (D–F) Cell division rate is decreased by dSkp2 knockdown (E, F), and GFP signals mark the RNAi-expressing clones (see the text for details), leading to smaller clones (arrows in E and F). In D, the GFP-marked cells are wild type. (D′–F′) Bar graphs showing the distribution of clones with different cell number per clone. N, number of total clones counted; M, median cell number per clone; DT, doubling time. The genotypes are y w, hs-flp/+; Act>y+>Gal4, UAS-GFP/+ (D and D’), y w, hs-flp/+; Act>y+>Gal4, UAS-GFP/dSkp2KK108837 (E, E′), and y w, hs-flp/+;Act>y+>Gal4, UAS-GFP/+; dSkp2GD5142/+ (F, F′). Scale bar, 25 μm.
Reduced cell density observed in Figure 4 can be explained by a defect in cell cycle progression caused by dSkp2 knockdown. An alternative (or additional) possibility is that a significant number of cells underwent apoptosis during wing development. It has been documented that cells containing a null allele of dSkp2, dSkp2ex9, exhibit enhanced apoptosis, in addition to other cell cycle defects (Ghorbani et al., 2011). The results shown in Figure 4, A–C, were obtained under a condition that resembles the null allele with respect to the presence of apoptotic cells induced by dSkp2 RNAi (data not shown). In these experiments, dicer2 was coexpressed with RNAi expression. We found that, without dicer2 coexpression, dSkp2 RNAi did not cause any detectable apoptosis (data not shown). To determine whether weaker perturbations caused by dSkp2 knockdown are sufficient to affect cell number without crossing the threshold of causing apoptosis, we performed a flip-out analysis (Neufeld et al., 1998) under the condition in which dicer2 was not coexpressed. As noted by Ghorbani et al. (2011), dSkp2 is located close to the centromere, precluding the use of mitotic recombination to generate dSkp2 mutant clones. Unlike the fluorescence-activated cell sorting technique, which monitors the snapshot behavior of a group of cells with regard to cell cycle progression, an advantage of the flip-out technique is that it quantifies the “accumulated” outcome of the behavior of cell cycle progression during a period of time. We reasoned that this “compounding effect” (due to time) may permit a sensitive detection of even potentially minor defects in cell cycle progression caused by weaker perturbations.
In our analysis, we used the flip-out technique to generate control (wild-type) or RNAi clones that are marked by green fluorescent protein (GFP; Figure 4, D–F; see Materials and Methods for details; Neufeld et al., 1998). We induced RNAi clones at 38 h after egg deposition (AED) and allowed animals to develop for another 77 h. We then counted the cells in both wild-type and dSkp2-knockdown clones. Figure 4, D′–F′, shows the distributions of clone size expressed as cell number in individual clones analyzed. The average clone sizes for wild-type, dSkp2KK108837, and dSkp2GD5142 clones were 143, 53, and 26 cells, respectively. On the basis of the measured clone size, we estimated the cell doubling time (see Materials and Methods). The estimated doubling times for wild-type cells, dSkp2KK108837 cells, and dSkp2GD5142 cells were 11, 13, and 16 h, respectively. These results support the suggestion that dSkp2 knockdown can prolong the cell cycle time of wing disc cells.
dSkp2 and dap have functions that intersect in controlling cell proliferation
If Dap is indeed a target of SCFdSkp2, patterning defects caused by reduced dSkp2 activity may be reflective of, at least in part, an increased Dap level in wing cells. To evaluate this possibility, we analyzed whether overexpression of dap can cause defects similar to those caused by dSkp2 knockdown. For this purpose, we used two independent transgenic lines, UAS-4xMyc-dap (see earlier discussion) and UAS-dapCL660 (Lane et al., 1996). Overexpression of dap in wing discs under the control of en-Gal4 led to a wing hair spacing phenotype (Figure 5A′′ and Supplemental Figure 3, C–C′ and D–D′′′′′). This phenotype is similar to that caused by dSkp2 knockdown, although with reduced severity (Figure 5A′; see Supplemental Figure S3 for high magnification). We also used other Gal4 drivers to further evaluate and confirm the phenotypic similarities between dSkp2 knockdown and dap overexpression. Figure 5, B′ and B′′, shows that dSkp2 knockdown or dap overexpression, driven by nub-Gal4 (in the entire wing), led to similar, although differing in severity, wing hair spacing phenotypes and partial loss of the wing margin. In addition, dSkp2 knockdown or dap overexpression, driven by vg-Gal4 at the D/V boundary of the wing disc, caused a similar wing margin loss phenotype (Figure 5, C and C′). Finally, notum-specific dSkp2 knockdown or dap overexpression, driven by pnr-Gal4, gave rise to similar dorsal closure defects and abnormal bristle patterning (Figure 5, D′ and D′′). Taken together, these results provide further support to the suggestion that dSkp2 and Dap have regulatory functions in a common pathway(s) during Drosophila development.
FIGURE 5:
Similar phenotypes caused by dap overexpression and dSkp2 knockdown. Light microscopic images showing wings (A, A′, and A′′; B, B′, and B′′; C, C′, and C′′) and nota (D, D′, and D′′) from flies with the indicated genotypes. (A) A control wing of en-Gal4. (A′, A′′) Wings exhibiting the WHS phenotype (with different severity) in the posterior regions, caused by either dSkp2 knockdown (A′) or dap overexpression (A′′), each under the control of en-Gal4. (B) A control wing of nub-Gal4. (B′, B′′) Wings showing the WHS phenotype in the entire wing by either dSkp2 knockdown (B′) or dap overexpression (B′′) under the control of nub-Gal4. (C) A control wing of vg-Gal4. (C′, C′′) Wings showing nicking phenotype on the wing margin (arrows), caused by either dSkp2 knockdown (C′) or dap overexpression (C′′) under the control of vg-Gal4. (D) A control notum of pnr-Gal4. (D′, D′′) Nota displaying dorsal closure defects and loss of bristles by either dSkp2 knockdown (D′) or overexpression of dap (D′′) under the control of pnr-Gal4.
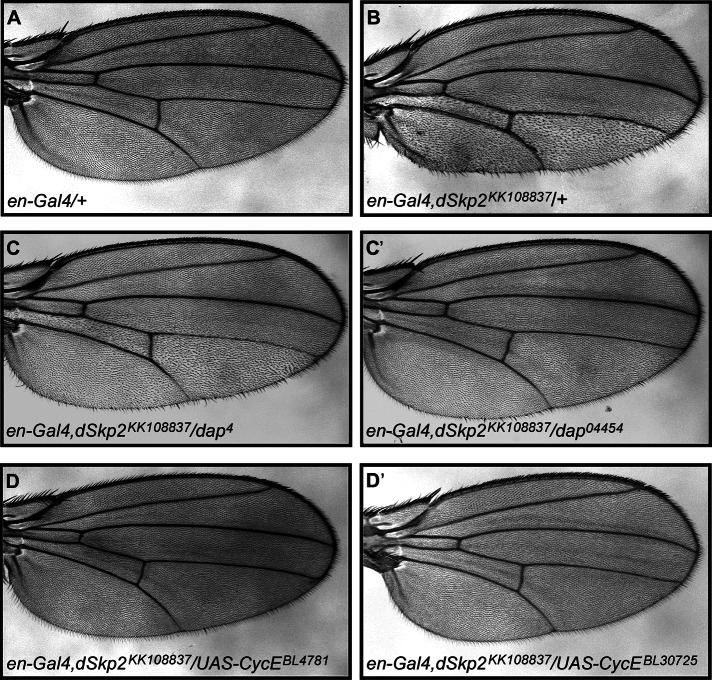
To further evaluate the functional relationship between dSkp2 and dap in vivo, we performed genetic interaction studies. Here one copy of the mutation, dap04454 or dap4, was introduced into flies that contain an en-Gal4, dSkp2KK10883 chromosome (Figure 6, C and C′). Our results show that reducing the dap gene dose by half was able to partially suppress the wing hair spacing phenotype caused by the en-Gal4, dSkp2KK108837 chromosome (compare Figure 6, C and C′ with B). These genetic interaction results show that dSkp2 and dap have functions that interact in controlling cell proliferation. This thus led us to examine whether dSkp2 could also genetically interact with other genes known to be involved in regulating Dap-mediated cell cycle progression.
FIGURE 6:
dSkp2 genetically interacts with dap and CycE in the Drosophila wing. (A) A control wing of en-Gal4. (B) dSkp2 knockdown in the posterior region of the wing under the control of en-Gal4 leads to the typical WHS phenotype. Introducing a mutant copy of either dap04454 (C) or dap4 (C′) into the en>dSkp2KK108837 flies partially suppresses the WHS phenotype. Overexpression of CycE from UAS-CycE (D, D′, two independent transgenes) almost completely rescues the WHS phenotype caused by en>dSkp2KK108837.
CycE and Cdk2 are two regulators that promote normal cell cycle in Drosophila (Sauer et al., 1995). Knocking down either CycE or Cdk2 in the wing led to a wing hair spacing phenotype similar to that caused by dSkp2 knockdown (Supplemental Figure S4, C and D). Of importance, overexpression of CycE almost completely suppressed the wing hair spacing phenotype caused by dSkp2 knockdown (Figure 6, D and D′). Taken together, these results show that dSkp2 genetically interacts with both dap and other regulatory genes important for cell proliferation, further supporting a role of dSkp2 in promoting cell cycle progression.
Evaluating the role of dSkp2 in Dap stability at a cellular level in fly tissues
The coIP studies and protein level measurements described in the foregoing suggested a role of dSkp2 in regulating Dap stability (Figures 1, D and E, and 2, B and C). Those experiments required the generation of extracts from cells or tissues and thus were not adequate to give insight into the role of dSkp2 in regulating Dap stability at a cellular level. To further investigate whether the cellular Dap level is subject to dSkp2 regulation, we performed immunostaining studies in the wing disc. We compared the posterior region of the wing discs with or without dSkp2 knockdown (driven by en-Gal4). Dap protein level is uniformly low in wing disc cells (de Nooij et al., 2000). However, dSkp2 knockdown led to elevated staining of Dap in selected individual cells (Supplemental Figure S5B′; see Supplemental Figure S5A′, control; Supplemental Figure S5C′ shows simultaneous knockdown of dSkp2 and Cks85A with an enhanced effect; see Discussion for further information).
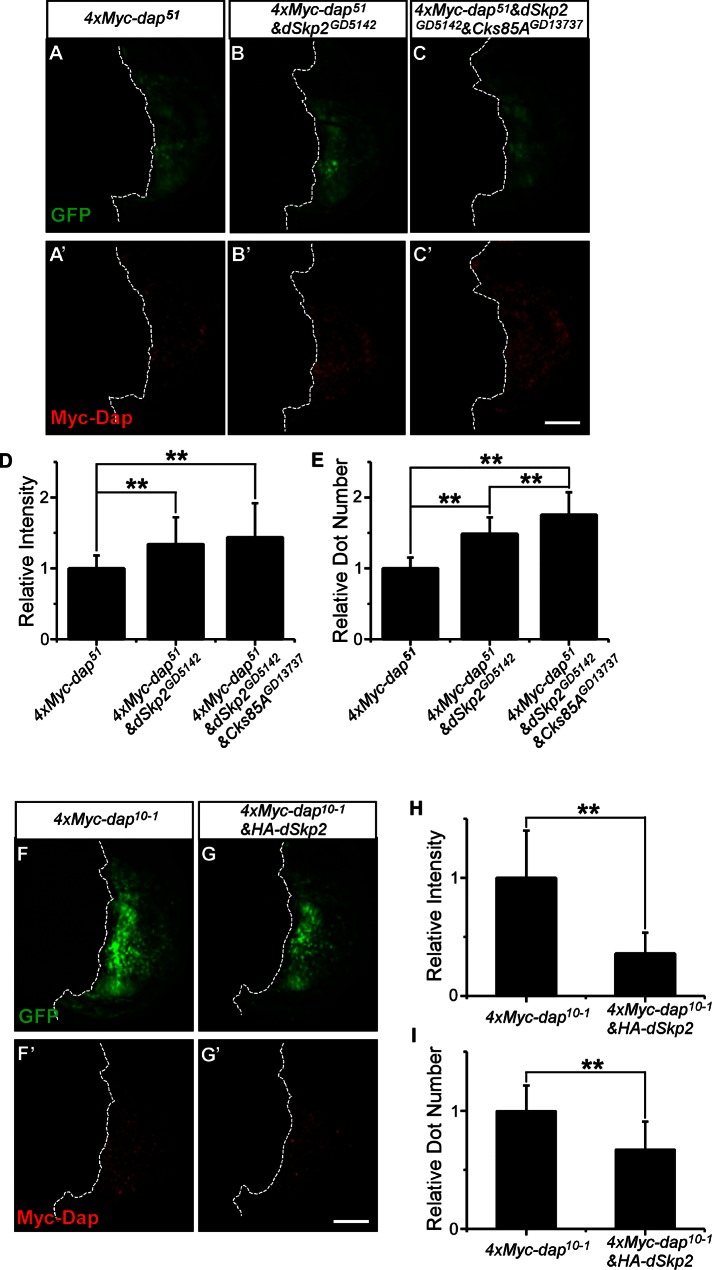
Low levels of the endogenous Dap in wild-type (wt) wing discs are difficult to detect by the antibody we used (de Nooij et al., 2000). In the experiments shown in Supplemental Figure S5, the uniformly low levels of Dap in wt wing discs are probably not beyond experimental background levels. This technical limitation is likely a contributing factor for the relatively modest effects of dSkp2 knockdown on Dap levels under the experimental conditions. To get around this technical limitation and increase the detection sensitivity for an improved evaluation of the role of dSkp2 in regulating Dap stability at a cellular level, we took advantage of UAS-4xMyc-dap transgenic lines and used an anti-Myc antibody in our immunostaining assays. We approached this problem by analyzing the effects of either knockdown or overexpression of dSkp2 on the detected signals for 4xMyc-Dap in wing discs. For each set of experiments, we ensured that all the experimental and imaging steps were performed side by side to allow effective comparisons. Figure 7, A′–C′, shows immunostained wing discs that express, respectively, 4xMyc-dap alone, 4xMyc-dap together with dSkp2 knockdown, and 4xMyc-dap together with dSkp2 and Cks85A knockdown. Expression of 4xMyc-Dap alone led to accumulations of the protein in a nonuniform manner among the wing disc cells (Figure 7A′). With dSkp2 knockdown, we detected a significant increase in both the mean number of fluorescence intensity clusters (referred to as intensity dots) within the 4xMyc-dap expression domain of the disc and the mean aggregate intensity of these dots (see Figure 7, D and E, for further increase caused by simultaneous knockdown of Cks85A). Conversely, coexpression of HA-dSkp2 led to significant reduction in these quantities (Figure 7, H and I). Taken together, these results provide further in vivo evidence supporting our suggestion that Dap is a substrate of SCFdSkp2 in manifesting the patterning defects caused by reduction in dSkp2 activity during development.
FIGURE 7:
dSkp2 has a role in regulating Dap protein level in vivo. (A–E) Knockdown of dSkp2 leads to an increase in fluorescence signals for 4xMyc-Dap in wing discs. 4xMyc-dap51 was expressed in the posterior regions of the wing discs under the control of en-Gal4 either alone (A, A′) or with the dSkp2 knockdown (B, B′) or with a simultaneous knockdown of dSkp2 and Cks85A (C, C′). The confocal images shown were from side-by-side experiments and were captured under identical imaging settings. (D, E) Mean number of clusters of fluorescence signals (referred to as dots) per disc and mean aggregate intensity (in arbitrary units), respectively, of the detected dots. Error bars, SD calculated across different discs in a group. n = 20 discs for each genotype. (F–I) Coexpression of HA-dSkp2 leads to a reduction in fluorescence signals for 4xMyc-Dap in wing discs. 4xMyc-dap10–1 was expressed in the posterior region of the wing discs under the control of en-Gal4 either alone (F, F′) or with HA-dSkp2 together (G, G′). The confocal images shown were based on side-by-side experiments with images captured under identical settings. (H,I) Mean number of fluorescent dots detected per disc and mean aggregate intensity (in arbitrary units) of the detected dots, respectively. n = 13 for 4xMyc-dap10–1 discs and 19 for 4xMyc-dap10–1&HA-dSkp2 discs. **p < 0.01 (Student's t test). Scale bar, 75 μm.
DISCUSSION
Despite extensive studies of human Skp2 since its discovery more than a decade ago, the role of Drosophila Skp2 (dSkp2) has not been fully characterized. It is logical to infer that, for a highly conserved and fundamental process such as cell cycle control, the basic structure of the regulatory networks is most likely conserved among different species. However, it is unresolved whether the functional relationship between dSkp2 and its inferred target Dap is indeed conserved (see Introduction). In particular, a recent study raised the question of whether dSkp2 has a role in regulating Dap stability (Ghorbani et al., 2011). Our study was designed to shed light on this specific question. We provide both in vitro and in vivo evidence that documents a role of dSkp2 in targeting Dap for ubiquitination and degradation and in regulating cell cycle progression. Our conclusion regarding the role of dSkp2 in regulating Dap stability differs from that of Ghorbani et al. (2011), likely reflecting differences in detection method or sensitivity, as opposed to the way in which dSkp2 is perturbed. The dSkp2-null allele and the RNAi lines used in this study had identical phenotypes and, of importance, the dSkp2-knockdown phenotypes can be fully rescued by wild-type dSkp2. Because Skp2 has been associated with multiple cancers (see Introduction), our study thus suggests that Drosophila as a model system may benefit future studies of Skp2-mediated human tumors (Gstaiger et al., 2001; Signoretti et al., 2002; Ben-Izhak et al., 2003; Wang XC et al., 2012; Wang Z et al., 2012).
The conservation between the Dap/p21-mediated cell cycle control networks in humans and flies extends to the function of Cdc kinase subunit 1 (Cks1) in regulating Dap/p21 protein level. Cks1 is a small accessory protein that facilitates SCFSkp2 in targeting its substrates for degradation (Ganoth et al., 2001; Mongay et al., 2001; Spruck et al., 2001; Wang et al., 2003, 2004; Hao et al., 2005; Yao et al., 2006). The Drosophila homologue of Cks1, Cks85A, was recently shown to interact with dSkp2 and play an important role in development (Ghorbani et al., 2011). In our study, knockdown of Cks85A and dSkp2 caused a similar wing hair spacing phenotype (Supplemental Figure S4B). Simultaneous knockdown of dSkp2 and Cks85A led to more pronounced accumulations of the endogenous Dap protein relative to dSkp2 or Cks85A knockdown alone (Supplemental Figure S5 and data not shown). Because the physical interaction between dSkp2 and Dap is enhanced by Cks85A (Figure 1A, lane 12), Cks85A most likely achieves its biological functions through a conserved molecular mechanism of strengthening the interaction between SCFSkp2 and its substrates (Ganoth et al., 2001; Spruck et al., 2001).
It is well documented that E3 ligases can have multiple substrate proteins and a given protein can be targeted for degradation by multiple E3 ligases (Skaar et al., 2009a, b; Dui et al., 2012). These particular features of the E3 ligase–substrate relationship contribute significantly to the richness of the biological regulatory systems. In addition to p21cip1/p27kip1/p57kip2, human Skp2 can also target other proteins for degradation, including CycA (Yam et al., 1999; Ji et al., 2006), CycD1 (Ganiatsas et al., 2001), Cdk9 (Kiernan et al., 2001), CycE (Yeh et al., 2001), pRbp130 (Tedesco et al., 2002; Bhattacharya et al., 2003), Myc (Kim et al., 2003; von der Lehr et al., 2003), and Cdt1 (Li et al., 2003). Thus it is likely that dSkp2 may also have multiple substrates to execute the various biological functions of dSkp2. For example, it remains possible that, in addition to Dap, dSkp2 may also have a role in regulating the activity and/or stability of Cdt1/Dup in Drosophila and contribute to the development of polyploidy cells. It has been reported that overexpression of Drosophila Cdt1/dup (double-parked) is sufficient to induce rereplication (Whittaker et al., 2000; Thomer et al., 2004; May et al., 2005; Lin et al., 2009). Future studies with sensitive tools are required to further investigate whether there might be a functional relationship between dSkp2 and Cdt1/dup and, if there is, the disentangled molecular and genetic relationships among all the relevant regulators in maintaining diploidy in mitotic cells (Ghorbani et al., 2011). We note that, despite the complexity of the regulatory roles of dSkp2 during development, an important finding of our study is that the defects in cell cycle progression and enhanced apoptosis are two aspects of altered cellular behavior that respond to two distinct thresholds of dSkp2 activity alterations in the wing disc (Figure 4).
In humans, p21cip1 has been shown to undergo proteasome-mediated degradation through the actions of at least two E3 ligases, CRL4Cdt2 and SCFSkp2. Whereas CRL4Cdt2 predominantly regulates p21 levels during the S phase, SCFSkp2-mediated degradation of p21 does not have such a temporal restriction. In Drosophila, Dap protein level is known to oscillate during ovarian endocycles (de Nooij et al., 2000). In our experiments, the heterogeneity in 4xMyc-Dap staining in the wing disc is not abolished by dSkp2 activity alterations (Figure 7), suggesting that Dap may also be targeted for degradation by multiple E3 ligases and that dSkp2 may not be primarily responsible for its oscillating property. Finally, the E3 ligase complex of Cul4-DDB1-Cdt2 is conserved in Drosophila (Zielke et al., 2011), and a loss of Cul-4 based E3 ligases causes a G1 arrest in a manner that is dependent on Dap (Higa et al., 2006). These results suggest that Dap stability may also be subject to regulation by Cul-4 E3 ligases. Understanding the complete fine structure of the regulatory networks for cell cycle control and their roles during development remains an interesting scientific problem and long-term challenge. The present study, which documents the dSkp2–Dap connection, represents an important step toward meeting this challenge through the use of a genetic model system.
MATERIALS AND METHODS
Drosophila strains used in this study are listed according to their sources (see foregoing text for references to individual stocks/mutations):
Vienna Drosophila RNAi Center (Vienna, Austria): dSkp2GD5142, dSkp2KK108837, Cks85AGD13737.
Bloomington Drosophila Stock Center (Bloomington, IN): w*;dap4/CyO, dap04454/CyO, dSkp2JF01326, dSkp2HMS00116, Cdk2HMS00174, w;UAS-CycE.L, w*;UAS-CycE.R, UAS-dcr2 w1118; en-Gal4 UAS-GFP, UAS-dcr2 w1118;nub-Gal4, UAS-dcr2 w1118;pnr-Gal4.
National Institute of Genetics (Mishima, Japan): CycE-IR.
Christian F. Lehner (University of Zurich, Switzerland): UAS-DapCL659 and UAS-DapCL660 transgenic flies.
Andrew Swan (University of Windsor, Canada): dSkp2ex9 mutant.
Flies were reared under standard condition at 25°C unless stated otherwise.
Generation of transgenic flies
To construct pUAST-dSkp2, two PCR fragments amplified from dSkp2/CG9772 genomic DNA were obtained using the following primers: 5′-ataagaatGCGGCCGCAGCGCGAAGTTTTTGTGTTT-3′ (NotI site in bold capitals, ATG codon of dSkp2 coding sequence is after this primer) and 5′-TGGAAGCCATACTCAGGTCA-3′ for fragment 1, 5′-TAAGTGGCTCCCGAAGAAGA-3′ and 5′-ctagTCTAGACGCAGCAAAACAAATTCAAA-3′ (XbaI site in bold capitals) for fragment 2. After cutting with NotI/XhoI and XhoI/XbaI, two fragments were ligated into the NotI/XbaI sites of the pUAST vector. To construct pUAST-HA-dSkp2, a modified pUAST vector with ATG start codon and HA tag sequence at the EcoRI site (Huang et al., 2011) was used. To construct the fusion gene encoding the HA-tagged dSkp2 protein, another pair of primers for PCR fragment 3 was used: 5′-ataagaatGCGGCCGCGGAGCAGTCGCAAGCACAG-3′ (NotI site in bold capitals) and 5′-TGGAAGCCATACTCAGGTCA-3′. Fragment 3, which was cut with NotI/XhoI, and the aforementioned fragment 2, which was cut with XhoI/XbaI, were ligated into NotI/XbaI linearized pUAST-HA vector. To construct pUAST-4xMyc-Dap, another modified pUAST vector (kindly provided by Jianming Chen, Xiamen University, China) with 4xMyc tag sequence inserted at BglII/KpnI site was used. A PCR fragment containing the entire coding region (except for the ATG start codon) was obtained using the following primers: 5′-ctagTCTAGAGGTCAGTGCCCGAGTCCT-3′ (XbaI site in bold capitals) and 5′-ctagTCTAGACCAGCGAATCTGGAGCATTA-3′ (XbaI site in bold capitals). After cutting with XbaI, the PCR fragment was cloned into the XbaI site of the pUAST-4xMyc vector, and clones with correct orientation were picked up for sequencing verification. Correct constructs with sequence confirmation of pUAST-dSkp2, pUAST-HA-dSkp2, and pUAST-4xMyc-Dap were used for microinjection to generate transgenic flies, using standard protocols (Xu et al., 2009; Chen et al., 2010).
Constructs for protein expression in S2 cells, transfection, and RNA interference assays
The pAc5.1/V5-His vector with the Drosophila actin 5C promoter was used for protein expression in S2 cells (Liu and Ma, 2011). Plasmids of pAc5.1-dSkp2-FLAG, pAc5.1-dSkp2-V5, pAc5.1–4xMyc-Dap, pAc5.1–4xMyc-SkpA, and pAc5.1-Cks85A-V5 were constructed by standard cloning methods (cloning strategies are available upon request). Drosophila S2 cells were cultured in Hyclone serum-free insect cell culture medium (Roche, Indianapolis, IN). Transfection of S2 cells was performed using FuGENE HD transfection reagent (Roche) according to the manufacturer's instructions. For treating S2 cells, the proteasome inhibitor epoxomicin (final concentration 300 nM; Sigma-Aldrich, St. Louis, MO), the lysosome inhibitor chloroquine diphosphate (final concentration 500 μM; Sigma-Aldrich), and the translation inhibitor cycloheximide (final concentration 100 μg/ml; Sigma-Aldrich) were used. For RNA interference, double-strand RNA (dsRNA) was prepared with the RiboMAX large-scale RNA Production System-T7 Kit (Promega, Madison, WI). The primers for GFP control were 5’-ttaatacgactcactataggggagaATGGTGAGCAAGGGCGAGGAGCTG-3′ (forward) and 5′-ttaatacgactcactataggggagaCTTGTACAGCTCGTCCATGCCGAGAG-3′ (reverse). The primers for dSkp2 RNAi were 5′-aattctaatacgactcactatagggagaCTCAATTTTCAGCCATCAACCAGCA-3′ (forward) and 5′-aattctaatacgactcactatagggagaCAAGGACTCCCCGCCGTACAA-3′ (reverse). For a 12-well plate, 1 ml of culture medium in each well was treated with 7.5 μg of dsRNA for 3 d before plasmid transfection.
Coimmunoprecipitation assays
S2 cells were transfected or cotransfected with plasmids that express a desired combination of proteins. Cell lysates were prepared in IP lysis buffer (50 mM Tris-HCl, 120 mM NaCl, 0.2 mM EDTA, 0.2 mM ethylene glycol tetraacetic acid, 0.4% NP40, 0.4% sodium deoxycholate), followed by ultrasonication treatment: 100 Hz, 4 s with 6-s interval, 10 times. For coIP assays with Drosophila tissue extracts, >500 adult fly heads were collected and homogenized in the same IP lysis buffer as used for the S2 cells. The prepared cell or tissue lysates were incubated with specific antibody and protein A/G agarose (Abmart, Shanghai, China) before proper centrifugation and buffer washes. Immunoprecipitates were resolved by SDS–PAGE, and Western blots were performed using appropriate antibodies according to standard protocols (Wei et al., 2008, 2010). Input lanes represent 1% of the total lysates used in coIP reactions unless stated otherwise. The loading of the immunoprecipitated products was identical for all lanes, each representing 15% of these products.
Immunocytochemistry
Wandering third-instar larvae with correct genotypes were collected and dissected in cold phosphate-buffered saline (PBS). Imaginal discs were fixed in 3.7% paraformaldehyde in PBS for 20 min. After four washes in PBS with 0.3% Triton X-100, 5% normal horse serum in PBS/Tween-20 was used for 15 min preblocking. The discs were then incubated with different primary antibodies (see later description) overnight at 4°C. Subsequently, corresponding fluorescent secondary antibodies were used for signal detection. All confocal images were captured using a Leica confocal microscope SP5 (Leica, Wetzlar, Germany) as described previously (Liu et al., 2011; Xie et al., 2012). All confocal images shown were captured under identical settings, each representing data from a single z-section.
Cell proliferation rate estimates
Wild-type, dSkp2KK108837, and dSkp2GD5142 clones were induced by the FRT (Flippase recognition target)-mediated “flip-out” method. The strain y w, hs-flp; Act>y+>Gal4, UAS-GFP was used for this purpose (Neufeld et al., 1998). Larvae of each genotype were heat shocked at 38 ± 1 h AED for 1 h at 37°C, and wing imaginal discs were dissected and fixed at 115 h AED (Neufeld et al., 1998). Clones marked by GFP-positive signals were imaged under a Leica confocal microscope SP5. Cells per clone were counted from 17 discs of wild type, 21 discs of dSkp2KK108837, and 13 discs of dSkp2GD5142. Median cell number per clone is taken as M, and cell doubling time (DT) was calculated as DT = T/log2M (T = 77 h in this study).
Primary and secondary antibodies
The primary antibodies were diluted to 1:100 for immunofluorescence assays and 1:5000 for Western blotting. Antibodies used in this study were as follows: rabbit anti–c-Myc (Sigma-Aldrich); mouse anti–c-Myc (CWBIO, Beijing, China); mouse anti-V5 (Invitrogen, Carlsbad, CA); mouse anti-HA (Abmart); mouse anti-Dap (1:5 dilution; DSHB-NP1; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); mouse anti-Flag (Sigma-Aldrich); and anti-tubulin (CWBIO). For Western blotting, horseradish peroxidase–linked secondary antibodies (1:5000 dilution) were used. For immunostaining assays, the corresponding fluorescent secondary antibodies (fluorescein isothiocyanate, tetramethylrhodamine isothiocyanate, or Cy5) were used in 1:400 dilution.
Quantification of fluorescence signals for 4xMyc-tagged Dap in wing discs
Quantification of the fluorescence signals for 4xMyc-tagged Dap was performed to evaluate both the number of fluorescent clusters (referred to as dots) in a disc and the aggregate intensity (in arbitrary units) of the detected dots. Briefly, for each image, we first used DAPI counterstain signals to mask the disc. Whenever necessary, we performed manual cropping to remove the conjugated, unwanted tissues or other discs present in an image. We then used the Otsu thresholding algorithm (Otsu, 1979; Cheung et al., 2011) to define a threshold. Clusters of fluorescence intensity signals above this threshold were identified, and the number was determined for each disc. The aggregate intensities (in arbitrary units) of individual dots were also calculated, and a mean was calculated for all the detected dots in each disc. All calculations were performed on MATLAB (MathWorks, Natick, MA).
Supplementary Material
Acknowledgments
We thank Christian F. Lehner, Andrew Swan, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, and the National Institute of Genetics for fly stocks, the Developmental Studies Hybridoma Bank for antibodies, and Jianming Chen for the pUAST-4xMyc vector. This work was supported financially by the 973 program (2009CB918702 and 2012CB825504), the National Natural Science Foundation of China (31071087 and 31271573) to R.J., and the National Science Foundation Beijing (5122027) to C.L. Work in J.M.’s lab was supported by grants from the National Institutes of Health (1R01GM101373) and National Science Foundation (IOS-0843424). We are grateful to the anonymous reviewers for constructive suggestions.
Abbreviations used:
- CDKs
cyclin-dependent protein kinases
- CHX
cycloheximide
- CKIs
CDK inhibitors
- Cks1
Cdc kinase subunit 1
- coIP
coimmunoprecipitation
- Dap
Dacapo
- DAPI
4′,6-diamidino-2-phenylindole
- dSkp2
DrosophilaSkp2
- dup
double-parked
- GFP
green fluorescent protein
- IB
immunoblotting
- RNAi
RNA interference
- wt
wild type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-10-0772) on April 3, 2013.
REFERENCES
- Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG Jr. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati B, Vlach J. Kip1 meets SKP2: new links in cell-cycle control. Nat Cell Biol. 1999;1:E91–E93. doi: 10.1038/12087. [DOI] [PubMed] [Google Scholar]
- Ben-Izhak O, Lahav-Baratz S, Meretyk S, Ben-Eliezer S, Sabo E, Dirnfeld M, Cohen S, Ciechanover A. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J Urol. 2003;170:241–245. doi: 10.1097/01.ju.0000072113.34524.a7. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Garriga J, Calbo J, Yong T, Haines DS, Grana X. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene. 2003;22:2443–2451. doi: 10.1038/sj.onc.1206339. [DOI] [PubMed] [Google Scholar]
- Bond M, Sala-Newby GB, Wu YJ, Newby AC. Biphasic effect of p21Cip1 on smooth muscle cell proliferation: role of PI 3-kinase and Skp2-mediated degradation. Cardiovasc Res. 2006;69:198–206. doi: 10.1016/j.cardiores.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dui W, Yu Z, Li C, Ma J, Jiao R. Drosophila RecQ5 is required for efficient SSA repair and suppression of LOH in vivo. Protein Cell. 2010;1:478–490. doi: 10.1007/s13238-010-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D, Miles C, Kreitman M, Ma J. Scaling of the Bicoid morphogen gradient by a volume-dependent production rate. Development. 2011;138:2741–2749. doi: 10.1242/dev.064402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Graber KH, Hariharan IK. Expression of the cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E. Mech Dev. 2000;97:73–83. doi: 10.1016/s0925-4773(00)00435-4. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Letendre MA, Hariharan IK. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- Dui W, Lu W, Ma J, Jiao R. A systematic phenotypic screen of F-box genes through a tissue-specific RNAi-based approach in Drosophila. J Genet Genomics. 2012;39:397–413. doi: 10.1016/j.jgg.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Follette PJ, O'Farrell PH. Cdks and the Drosophila cell cycle. Curr Opin Genet Dev. 1997;7:17–22. doi: 10.1016/s0959-437x(97)80104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganiatsas S, Dow R, Thompson A, Schulman B, Germain D. A splice variant of Skp2 is retained in the cytoplasm and fails to direct cyclin D1 ubiquitination in the uterine cancer cell line SK-UT. Oncogene. 2001;20:3641–3650. doi: 10.1038/sj.onc.1204501. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Vasavan B, Kraja E, Swan A. Cks85A and Skp2 interact to maintain diploidy and promote growth in Drosophila. Dev Biol. 2011;358:213–223. doi: 10.1016/j.ydbio.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao B, Zheng N, Schulman BA, Wu G, Miller JJ, Pagano M, Pavletich NP. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell. 2005;20:9–19. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Higa LA, Yang X, Zheng J, Banks D, Wu M, Ghosh P, Sun H, Zhang H. Involvement of CUL4 ubiquitin E3 ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E degradation. Cell Cycle. 2006;5:71–77. doi: 10.4161/cc.5.1.2266. [DOI] [PubMed] [Google Scholar]
- Huang H, Du G, Chen H, Liang X, Li C, Zhu N, Xue L, Ma J, Jiao R. Drosophila Smt3 negatively regulates JNK signaling through sequestering Hipk in the nucleus. Development. 2011;138:2477–2485. doi: 10.1242/dev.061770. [DOI] [PubMed] [Google Scholar]
- Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- Ji P, Goldin L, Ren H, Sun D, Guardavaccaro D, Pagano M, Zhu L. Skp2 contains a novel cyclin A binding domain that directly protects cyclin A from inhibition by p27Kip1. J Biol Chem. 2006;281:24058–24069. doi: 10.1074/jbc.M603105200. [DOI] [PubMed] [Google Scholar]
- Kiernan RE, Emiliani S, Nakayama K, Castro A, Labbe JC, Lorca T, Nakayama Ki K, Benkirane M. Interaction between cyclin T1 and SCF(SKP2) targets CDK9 for ubiquitination and degradation by the proteasome. Mol Cell Biol. 2001;21:7956–7970. doi: 10.1128/MCB.21.23.7956-7970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell. 2003;11:1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- Kossatz U, Dietrich N, Zender L, Buer J, Manns MP, Malek NP. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev. 2004;18:2602–2607. doi: 10.1101/gad.321004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell. 1996;87:1225–1235. doi: 10.1016/s0092-8674(00)81818-8. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Q, Liao R, Sun P, Wu X. The SCF(Skp2) ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J Biol Chem. 2003;278:30854–30858. doi: 10.1074/jbc.C300251200. [DOI] [PubMed] [Google Scholar]
- Lin HC, Wu JT, Tan BC, Chien CT. Cul4 and DDB1 regulate Orc2 localization, BrdU incorporation and Dup stability during gene amplification in Drosophila follicle cells. J Cell Sci. 2009;122:2393–2401. doi: 10.1242/jcs.042861. [DOI] [PubMed] [Google Scholar]
- Liu J, Ma J. Fates-shifted is an F-box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos. Nat Cell Biol. 2011;13:22–29. doi: 10.1038/ncb2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu Q, He D, Ma T, Du L, Dui W, Guo X, Jiao R. Drosophila sbo regulates lifespan through its function in the synthesis of coenzyme Q in vivo. J Genet Genomics. 2011;38:225–234. doi: 10.1016/j.jgg.2011.05.002. [DOI] [PubMed] [Google Scholar]
- May NR, Thomer M, Murnen KF, Calvi BR. Levels of the origin-binding protein Double parked and its inhibitor Geminin increase in response to replication stress. J Cell Sci. 2005;118:4207–4217. doi: 10.1242/jcs.02534. [DOI] [PubMed] [Google Scholar]
- Moberg KH, Mukherjee A, Veraksa A, Artavanis-Tsakonas S, Hariharan IK. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol. 2004;14:965–974. doi: 10.1016/j.cub.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Mongay L, Plaza S, Vigorito E, Serra-Pages C, Vives J. Association of the cell cycle regulatory proteins p45(SKP2) and CksHs1. Functional effect on CDK2 complex formation and kinase activity. J Biol Chem. 2001;276:25030–25036. doi: 10.1074/jbc.M102184200. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Morisaki H, Fujimoto A, Ando A, Nagata Y, Ikeda K, Nakanishi M. Cell cycle-dependent phosphorylation of p27 cyclin-dependent kinase (Cdk) inhibitor by cyclin E/Cdk2. Biochem Biophys Res Commun. 1997;240:386–390. doi: 10.1006/bbrc.1997.7590. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- Pagano M. Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Mol Cell. 2004;14:414–416. doi: 10.1016/s1097-2765(04)00268-0. [DOI] [PubMed] [Google Scholar]
- Pateras IS, et al. Downregulation of the KIP family members p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119:2546–2556. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- Sauer K, Knoblich JA, Richardson H, Lehner CF. Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev. 1995;9:1327–1339. doi: 10.1101/gad.9.11.1327. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Di Marcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M, Pagano M. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–641. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Skaar JR, D'Angiolella V, Pagan JK, Pagano M. SnapShot: F box proteins II. Cell. 2009a;137:1358, 1358e1. doi: 10.1016/j.cell.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Skaar JR, Pagan JK, Pagano M. SnapShot: F box proteins I. Cell. 2009b;137:1160–1160e1. doi: 10.1016/j.cell.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- Swan A, Schupbach T. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development. 2007;134:891–899. doi: 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer M, May NR, Aggarwal BD, Kwok G, Calvi BR. Drosophila double-parked is sufficient to induce re-replication during development and is regulated by cyclin E/CDK2. Development. 2004;131:4807–4818. doi: 10.1242/dev.01348. [DOI] [PubMed] [Google Scholar]
- Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Lehr N, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Wang W, Ungermannova D, Chen L, Liu X. A negatively charged amino acid in Skp2 is required for Skp2-Cks1 interaction and ubiquitination of p27Kip1. J Biol Chem. 2003;278:32390–32396. doi: 10.1074/jbc.M305241200. [DOI] [PubMed] [Google Scholar]
- Wang W, Ungermannova D, Chen L, Liu X. Molecular and biochemical characterization of the Skp2-Cks1 binding interface. J Biol Chem. 2004;279:51362–51369. doi: 10.1074/jbc.M405944200. [DOI] [PubMed] [Google Scholar]
- Wang XC, Tian LL, Tian J, Jiang XY. Overexpression of SKP2 promotes the radiation resistance of esophageal squamous cell carcinoma. Radiat Res. 2012;177:52–58. doi: 10.1667/rr2679.1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, Sarkar FH, Wei W. Skp2: A novel potential therapeutic target for prostate cancer. Biochim Biophys Acta. 2012;1825:11–17. doi: 10.1016/j.bbcan.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Chen Z, Zhang X, Feldman M, Dong XZ, Doran R, Zhao BL, Yin WX, Kotlikoff MI, Ji G. Nitric oxide mediates stretch-induced Ca2+ release via activation of phosphatidylinositol 3-kinase-Akt pathway in smooth muscle. PLoS One. 2008;3:e2526. doi: 10.1371/journal.pone.0002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Gao J, Huang XP, Jin JP. Mutual rescues between two dominant negative mutations in cardiac troponin I and cardiac troponin T. J Biol Chem. 2010;285:27806–27816. doi: 10.1074/jbc.M110.137844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann K, Cohen SM, Lehner CF. Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development. 1997;124:3555–3563. doi: 10.1242/dev.124.18.3555. [DOI] [PubMed] [Google Scholar]
- Whittaker AJ, Royzman I, Orr-Weaver TL. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- Wojda I. The group of protein kinases CKI [in Polish] Postepy Biochem. 2000;46:140–147. [PubMed] [Google Scholar]
- Xie G, Zhang H, Du G, Huang Q, Liang X, Ma J, Jiao R. Uif, a large transmembrane protein with EGF-like repeats, can antagonize Notch signaling in Drosophila. PLoS One. 2012;7:e36362. doi: 10.1371/journal.pone.0036362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lei Z, Huang H, Dui W, Liang X, Ma J, Jiao R. dRecQ4 is required for DNA synthesis and essential for cell proliferation in Drosophila. PLoS One. 2009;4:e6107. doi: 10.1371/journal.pone.0006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam CH, Ng RW, Siu WY, Lau AW, Poon RY. Regulation of cyclin A-Cdk2 by SCF component Skp1 and F-box protein Skp2. Mol Cell Biol. 1999;19:635–645. doi: 10.1128/mcb.19.1.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZP, Zhou M, Kelly SE, Seeliger MA, Robinson CV, Itzhaki LS. Activation of ubiquitin ligase SCF(Skp2) by Cks1: insights from hydrogen exchange mass spectrometry. J Mol Biol. 2006;363:673–686. doi: 10.1016/j.jmb.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Yeh KH, Kondo T, Zheng J, Tsvetkov LM, Blair J, Zhang H. The F-box protein SKP2 binds to the phosphorylated threonine 380 in cyclin E and regulates ubiquitin-dependent degradation of cyclin E. Biochem Biophys Res Commun. 2001;281:884–890. doi: 10.1006/bbrc.2001.4442. [DOI] [PubMed] [Google Scholar]
- Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y, Walker JL, Roberts JM, Assoian RK. A Skp2 autoinduction loop and restriction point control. J Cell Biol. 2007;178:741–747. doi: 10.1083/jcb.200703034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. Skp2 knockout reduces cell proliferation and mouse body size: and prevents cancer. Cell Res. 2010;20:605–607. doi: 10.1038/cr.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke N, et al. Control of Drosophila endocycles by E2F and CRL4(CDT2) Nature. 2011;480:123–127. doi: 10.1038/nature10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.