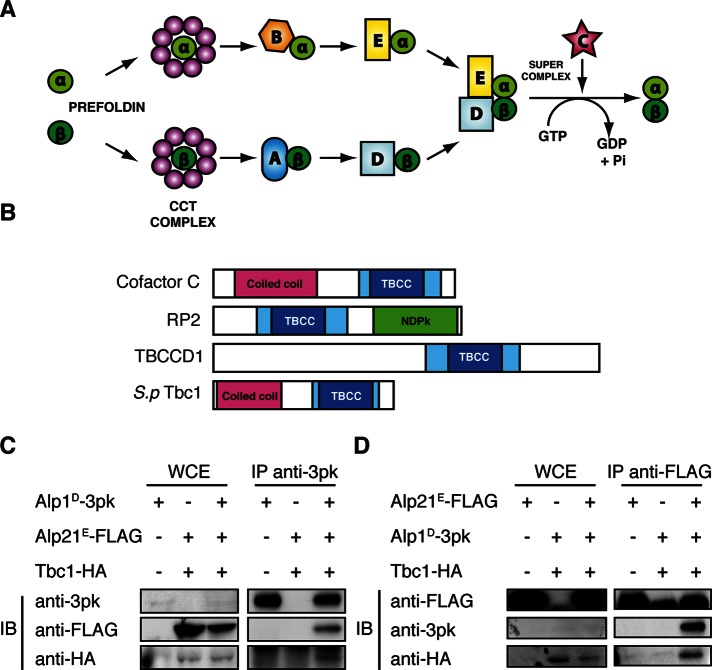
FIGURE 1:
Fission yeast Tbc1 is a conserved orthologue of tubulin-folding cofactor C. (A) Model of the canonical tubulin-folding pathway. The native α- and β-tubulin monomers are folded into functional heterodimers by a specific set of chaperones, cofactors A–E. (B) The conserved domains of the human TBCC domain containing proteins and fission yeast Tbc1. The TBCC domain is shown in blue, with the dark blue indicating two CARP domains usually found in CAP proteins. The coiled-coil regions shown in magenta suggest a protein–protein interaction domain, and the nucleotide diphosphate kinase (NDPK) shown in green is a phosphocarrier domain. (C, D) Immunoprecipitation of Alp1D-3Pk, Alp21E-3FLAG, and Tbc1–3HA. The immunoblot from IP against an anti-Pk antibody and an anti-FLAG antibody is shown in C and D, respectively. In both blots, the first lane shows the single-tagged strain and the second lane the doubly tagged strain. The third lane on the far right is the result from the triply tagged strain. For each IP 5 mg of protein was used, and 30 μg of whole-cell extract was loaded as a control.