Figure 2. Antigen specificity is required for the protection induced by PLP139–151 16-mer.
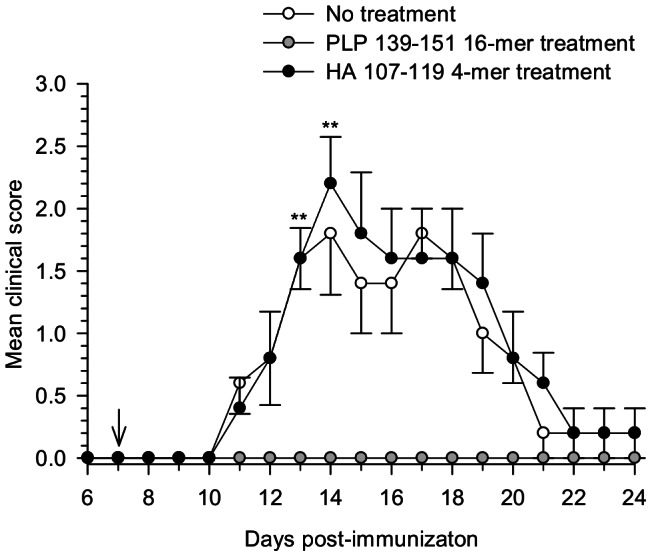
EAE was induced in SJL/J mice with the PLP139–151 peptide in complete adjuvant. On day 7 after disease induction, mice were treated intravenously with PLP139–151 16-mer or with an unrelated oligomer containing an epitope derived of the influenza virus hemagglutinin protein (HA107–119 4-mer) as indicated by the arrow. Mice were monitored daily and the average ±SEM of the clinical score of five mice per group was calculated. Statistical significant difference between the PLP139–151 16-mer and HA107–119 4-mer treated groups was observed (**P<0.01).
