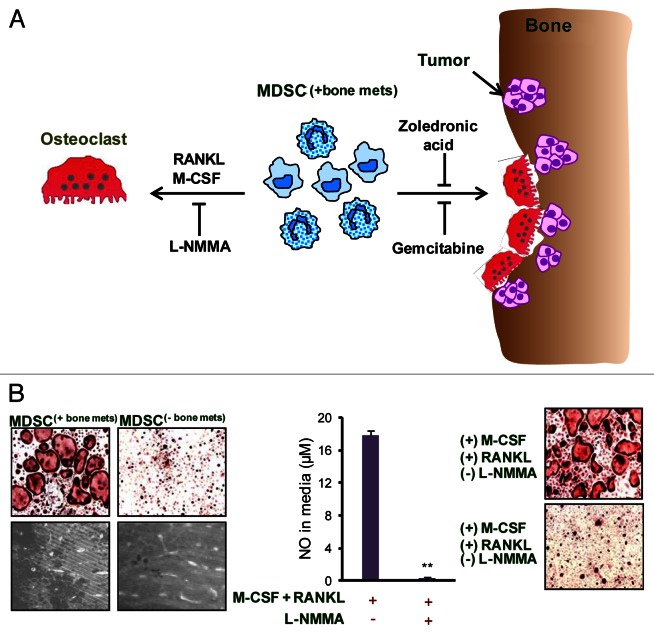
Figure 1. Role and mechanism of MDSC-mediated osteoclastogenesis in bone metastases. (A) As osteolytic cancers grow within bone, an increased influx of myeloid derived suppressor cells (MDSC(+bone mets)) is noted. When these MDSC(+bone mets) are cultured in the presence of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), they undergo differentiation into multi-nucleated, acid phosphatase 5, tartrate resistant (ACP5)+ osteoclasts. Nitric oxide (NO) signaling is crucial for MDSC differentiation into osteoclasts and can be blunted using NG-monomethyl-L-arginine acetate (L-NMMA), an inducible NO synthase (iNOS) inhibitor. The adoptive transfer of MDSC(+bone mets) results in increased osteolytic lesions and tumor growth in the bone of recipient mice. MDSC-mediated osteolysis can also be controlled in vivo by gemcitabine or zoledronic acid. The administration of either these agents reduces MDSC abundance, hence limiting osteolytic lesions and tumor growth. (B) Data show that MDSC isolated from mice bearing bone metastases (MDSC(+bone mets)) are capable of differentiating into osteoclasts and resorbing bone, whereas MDSC from mice bearing non-metastatic tumors (MDSC(-bone mets)) failed to differentiate into osteoclasts or exert bone-resorbing functions. NO levels are elevated in MDSC(+bone mets) and NO production can be inhibited with L-NMMA. In the presence of L-NMMA, MDSC(+bone mets) fail to differentiate into osteoclasts, proving the critical role of NO in this process.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.