Abstract
Neuroinflammation, through production of proinflammatory molecules and activated glial cells, is implicated in Alzheimer's disease (AD) pathogenesis. One such proinflammatory mediator is tumor necrosis factor α (TNF-α), a multifunctional cytokine produced in excess and associated with amyloid β–driven inflammation and cognitive decline. Long-term global inhibition of TNF receptor type I (TNF-RI) and TNF-RII signaling without cell or stage specificity in triple-transgenic AD mice exacerbates hallmark amyloid and neurofibrillary tangle pathology. These observations revealed that long-term pan anti–TNF-α inhibition accelerates disease, cautions against long-term use of anti–TNF-α therapeutics for AD, and urges more selective regulation of TNF signaling. We used adeno-associated virus vector–delivered siRNAs to selectively knock down neuronal TNF-R signaling. We demonstrate divergent roles for neuronal TNF-RI and TNF-RII where loss of opposing TNF-RII leads to TNF-RI–mediated exacerbation of amyloid β and Tau pathology in aged triple-transgenic AD mice. Dampening of TNF-RII or TNF-RI+RII leads to a stage-independent increase in Iba-1–positive microglial staining, implying that neuronal TNF-RII may act nonautonomously on the microglial cell population. These results reveal that TNF-R signaling is complex, and it is unlikely that all cells and both receptors will respond positively to broad anti–TNF-α treatments at various stages of disease. In aggregate, these data further support the development of cell-, stage-, and/or receptor-specific anti–TNF-α therapeutics for AD.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Alzheimer's disease (AD) pathophysiology is described by chronic and progressive neurodegeneration involving the genesis of extracellular amyloid β (Aβ) plaques, intraneuronal filamentous inclusions called neurofibrillary tangles (NFTs), synapse loss, inflammation, and neuronal cell death, ultimately leading to severe memory loss and cognitive impairment. Neuroinflammation is a highly enigmatic process contributing to disease pathogenesis in AD, where elevated levels of proinflammatory molecules have been associated with Aβ-induced inflammation, neurotoxicity, and cognitive decline.1–4 In AD-afflicted brains, microglia intimately co-localize with Aβ plaques and serve as major sources of proinflammatory mediators, including cytokines and chemokines.5 The pleiotropic proinflammatory cytokine tumor necrosis factor α (TNF-α) is produced in excess concurrently with increased Aβ plaque deposition, an observation that suggests that TNF-α levels reflect the pathologic progression of AD.6–8 Moreover, three TNF-α promoter polymorphisms have been associated with late-onset AD, and two of the three polymorphisms are linked to increased TNF-α production, further connecting this cytokine to the exacerbated chronic inflammatory disease status in AD.9 We and others have demonstrated that TNF-α expression is enhanced in AD mouse models where TNF-α is prepathologically up-regulated in 6-month-old triple-transgenic AD (3xTg-AD) mice,10,11 which corresponds with an enhancement of F4/80-positive microglial cell numbers.12 In addition, when neuron-specific TNF-α is chronically overexpressed in 3xTg-AD mice using adeno-associated virus (AAV) vectors, there is increased severity of inflammation, intracellular Aβ, and Tau pathology that leads to neuronal cell death portending that excessive and unopposed TNF-α signaling enhances AD-associated pathology and is detrimental to neuronal viability.13
TNF-α signals through two cognate transmembrane receptors, TNF receptor type I (TNF-RI) and TNF-RII, which are differentially expressed and regulated. TNF-RI is expressed constitutively on most cell types, whereas TNF-RII expression is induced and is restricted to specific cell populations, including hematopoietic cells, microglia, neurons, and endothelial cells.14,15 TNF-R engagement to its ligand mediates distinct cellular responses through the activation of several downstream signal transduction cascades involving the NFκB and JNK pathways. In the context of AD, several reports demonstrate differential roles and activation of TNF-RI and TNF-RII such that genetic deletion of TNF-RI, but not TNF-RII, results in reduced plaque deposition in the APP23 mouse model.16 Moreover, in human brain tissue, TNF-RI protein levels are increased, whereas TNF-RII levels are reduced in patients with AD relative to nondemented control brain.17 Taken together, these data imply an overall negative role for excessive TNF signaling on AD pathophysiology but, perhaps more importantly, illustrate the complexity of this signaling pathway.
Despite a large body of literature indicating detrimental roles for TNF-α, neuroprotective effects have also been reported. Early experiments revealed that TNF-α is protective in cultured neurons during glucose deprivation–induced injury and excitotoxicity by preserving Ca2+ homeostasis.18 Barger et al19 further demonstrated in dissociated neuronal cultures that pretreatment with TNF-α and Aβ peptide spares cells from Aβ-induced neuronal death, iron toxicity, and intracellular Ca2+ accumulation via an NF-κB–dependent mechanism. Moreover, neurons are vulnerable to ischemic injury and oxidative stress in TNF-R null mice, indicating that TNF-α is protective.20 Mice lacking TNF-R expression exhibited reduced manganese superoxide dismutase activity and lacked a robust microglial response to kainic acid.20 Similarly, cultured neurons pretreated with TNF-α resulted in a significant increase in manganese superoxide dismutase activity and a reduction in superoxide accumulation.21 These data add to the complexity of the TNF signaling pathway and suggest that strategies to modulate TNF-α in the disease setting may require selective tuning and specificity to ensure that protective signaling outcomes are not compromised.
Nonetheless, given the compelling data supporting the pathologic role of TNF-α in AD, the potential of using anti–TNF-α therapeutics has become a viable strategy for subverting the disease course. Preclinical data by McAlpine et al22 demonstrate that transiently inhibiting soluble TNF signaling in the 3xTg-AD mouse model using a dominant-negative inhibitor in conjunction with enhanced systemic inflammation prevents AD-associated amyloid pathology. Tobinick et al23 reported in a short-term, prospective, open-label pilot study that semiweekly perispinal administration of etanercept, a receptor decoy biological agent antagonizing the actions of TNF-α, in 15 patients with mild to severe AD led to significant and rapid cognitive improvements compared with untreated control patients as assessed by three separate tests measuring cognitive function.
Although previous studies provide evidence suggesting that TNF-α inhibition in the short-term may lead to improved pathologic and functional outcomes, they lack data addressing the long-term consequences of blocking TNF-α in a global manner, where cell, stage, and receptor specificity were not examined. To this end, we recently demonstrated that long-term global inhibition of TNF-R signaling in 3xTg-AD mice where TNF-RI and TNF-RII were ablated in all cell types results in a robust increase in hallmark amyloid and NFT pathology. Furthermore, in the absence of TNF signaling, microglia seem nonresponsive to the developing amyloid pathology, which correlates with an impairment of microglial-mediated Aβ42 phagocytosis activity in vitro.24 These data suggest that caution should be taken with the use of broad long-term anti-TNF inhibitors and that a more selective strategy should be investigated.
To add to our understanding of TNF signaling biology and the consequences of selectively modulating this pathway, we investigated the cell- and stage-specific role of TNF-R signaling in AD by using recombinant AAV (rAAV) vector–delivered siRNA technology to selectively knock down neuronal TNF-R signaling at stages preceding progressive pathology or in the presence of extant disease using the 3xTg-AD mouse model. We demonstrate that neuronal TNF-RI and TNF-RII exert differential actions where intact TNF-RII signaling results in suppressed Aβ plaque deposition and paired helical filament (PHF) formation in the context of progressive and established disease pathogenesis. In addition, we report a substantial reduction in Iba-1–positive microglia when rAAV2-delivered siTNF-RII or siTNF-RI+RII viral vectors are administered at 2 and 12 months of age. Taken together, these data demonstrate that selectively suppressing neuronal TNF-RI and/or TNF-RII leads to distinct and significant changes in AD pathogenesis, which is most likely a consequence of the divergent signaling pathways associated with these receptors. The present findings support further development and rigorous study of highly selective strategies designed to inhibit specific TNF-α–mediated signals and potentially disrupt the onset and/or progression of this debilitating disease.
Materials and Methods
Design, Verification, and Packaging of siTNF-RI–, siTNF-RII–, and siScr-Expressing rAAV2 Vectors
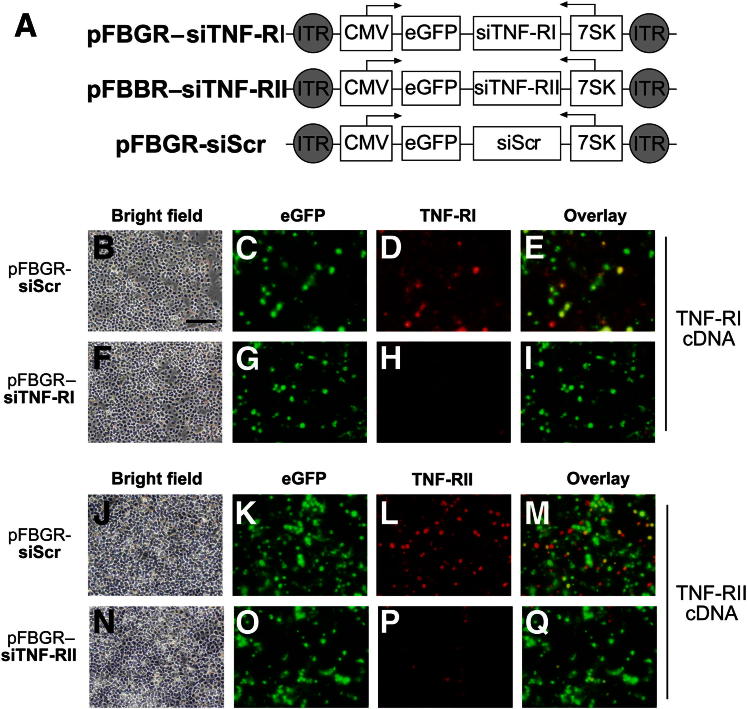
Using the siRNA Wizard algorithm (InvivoGen, San Diego, CA), target sequences for siRNA-mediated gene silencing were generated for TNF-RI and TNF-RII mRNA based on the following GenBank accession numbers: NM_011609 for TNF-RI and NM_011610 for TNF-RII. A scrambled control (Scr) sequence was also designed that lacked specificity to any mouse gene and served as a nonspecific negative control for subsequent experiments. Using the selected sequences, oligonucleotide pairs were designed to create a hairpin insert for plasmid siRNA (psiRNA)–7SKneo cloning vectors (InvivoGen). The RNA Pol III 7SK promoter and oligonucleotide sequence targeting each gene of interest were excised using the ClaI and XbaI restriction sites from the three custom-made psiRNA plasmids denoted as psiRNA–7SKneo–mTNF-RI, psiRNA–7SKneo–mTNF-RII, and psiRNA-7SKneo-mScr and cloned into the pBluescript II KS+ (pFBIIKS+) plasmid harboring a cytomegalovirus (CMV) promoter driving the expression of enhanced green fluorescent protein (eGFP) in the reverse orientation. This yielded intermediate plasmids containing a dual promoter system where the 7SK siRNA promoter drove the expression of siTNF-RI, siTNF-RII, and siScr, whereas in the opposite orientation, the CMV immediate-early promoter controlled eGFP expression. The resultant plasmids were designated pFBIIKS+(CMVeGFP rev)(7SK-mTNF-RI for), pFBIIKS+(CMVeGFP rev)(7SK-mTNFR-II for), and pFBIIKS+(CMVeGFP rev)(7SK-mTNF-R Scr for). The 7SK-siRNA and CMVeGFP transcriptional units were removed via NotI restriction digestion and were subcloned into a modified inverted terminal repeat–containing pFBGR plasmid lacking the eGFP gene (Figure 1A). The pFBGR(CMVeGFP rev)(mTNF-RI for), pFBGR(CMVeGFP rev)(mTNF-RII for), and pFBGR(CMVeGFP rev) (mTNFR Scr. for) plasmids were tested in vitro via transient transfection into baby hamster kidney (BHK) 21 cells. Knockdown efficiency was assessed by immunocytochemical analysis (Figure 1, B–Q) before packaging into serotype 2 AAV virions using a baculovirus-based procedure.25
Figure 1.
Construction and in vitro characterization of three rAAV vectors expressing siTNF-RI, siTNF-RII, or siScr. A: Schematic depicting a dual-promoter system of the rAAV2 constructs under the transcriptional regulation of the 7SK RNA polymerase III promoter encoding for siTNF-RI, siTNF-RII, or siScr and eGFP under the transcriptional control of the CMV immediate-early promoter in the reverse orientation. ITR, inverted terminal repeat. Before AAV2 packaging, BHK cells were transiently cotransfected with the pFBGR shuttle vector plasmids containing TNF-RI (B–I) or TNF-RII (J–Q) cDNA and the siTNF-RI (F–I), siTNF-RII (N–Q), or siScr (B–E and J–M) sequences. Cells were harvested 48 hours after transfection, and in vitro expression and receptor knockdown efficiency were assessed via IHC analysis using epifluorescence eGFP (C, G, K, and O) and mouse anti–TNF-RI (D and H) or mouse anti–TNF-RII (L and P) in conjunction with an Alexa Fluor 568 fluorescent secondary antibody (D, H, L, and P) for receptor expression detection. Bright field images were acquired at ×20 magnification (B, F, J, and N), and E, I, M, and Q represent merged eGFP and Alexa Fluor 568 signals. Scale bar = 200 μm.
Cell Transfections
BHK21 cells were plated at 5 × 104 cells per well in a poly-d-lysine–coated 24-well culture dish for immunocytochemical analysis. Transfections were performed using Lipofectamine 2000 (Life Technologies, Grand Island, NY) at equimolar quantities of plasmid: pFBGR-siScr + TNF-RI, pFBGR-siScr + TNF-RII, pFBGR-siTNF-RI + TNF-RI, or pFBGR–TNF-RII + TNF-RII cDNA. Cells were incubated with Opti-MEM (Life Technologies) serum-free media containing Lipofectamine and plasmid DNA for 5 hours at 37°C. Next, transfection medium was removed, and Dulbecco's modified Eagle's medium + 10% fetal bovine serum growth medium was added to the cells. Cells were fixed with 4% paraformaldehyde 48 hours after transduction.
Fluorescence Immunocytochemical Analysis
Paraformaldehyde-fixed cells were blocked with 10% normal goat serum in 1x Tris-buffered saline for 1 hour at room temperature. TNF-RI/RII expression was detected with polyclonal rabbit TNF-RI or TNF-RII primary antibodies (dilution 1:250; Abcam Inc., Cambridge, MA) in conjunction with Alexa Fluor 568 (dilution 1:200; Life Technologies, Grand Island, NY), whereas eGFP epifluorescence was used to determine transfection efficiency. Images were visualized via fluorescence microscopy at ×20 magnification.
Transgenic Mice
3xTg-AD mice of the B1 line were a gift from Dr. Frank LaFerla (University of California, Irvine, CA).10,11 Homozygous 3xTg-AD male mice were stereotactically injected with rAAV2 vectors using convection-enhanced delivery and were subsequently analyzed by immunohistochemical (IHC)/quantitative assessment studies (n = 6 per experimental group) or biochemical assays (n = 4 to 6). Mice were euthanized at 15 or 21 months of age. For IHC experiments, mice were sacrificed using pentobarbital overdose, followed by transcardiac perfusion with heparinized saline, followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. Brains were then sequentially transferred to 20% sucrose in 0.1 mol/L PBS overnight and 30% sucrose in 0.1 mol/L PBS. Next, 30-μm-thick sections were processed using a freezing stage microtome (Microm International, Walldorf, Germany) and were stored at −20°C in cryoprotectant until further use. For biochemical analyses, brains were microdissected from a separate cohort of mice to generate protein and RNA samples. Animal housing and procedures were performed in compliance with NIH Guidelines for Animal Care and Use and were approved by the University Committee of Animal Resources at the University of Rochester (Rochester, NY). Mice were on a 12-hour light-dark cycle in a climate-controlled facility and were allowed food and water ad libitum.
Stereotactic Injections by Convection-Enhanced Delivery of rAAV2-siRNAs
3xTg-AD male mice, 2 and 12 months old, stereotactically received bilateral hippocampal injections via convection-enhanced delivery using rAAV2-siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or an equal viral load of rAAV2–siTNF-RI+RII capsids in accordance with approved University of Rochester animal use guidelines as previously described.26 Briefly, under Avertin anesthesia (300 mg/kg) (Sigma-Aldrich, St. Louis, MO), mice were placed in a stereotactic apparatus, the skull was exposed, and two burr holes were drilled bilaterally at designated hippocampal coordinates (bregma position −2.06 mm, 1.5 mm laterally, −1.25 mm ventrally). Injections were performed using a microprocessor-controlled pump (UltraMicroPump; World Precision Instruments, Sarasota, FL) and a 33-gauge needle (Hamilton Co., Reno, NV) that was gradually lowered into the parenchyma to the desired depth. A total of 5 μL of each rAAV2-siRNA vector (2 × 109 transduction units) was continuously injected by increasing delivery rates at 100 nL/min for 6 minutes, 200 nL/min for 10 minutes, and 400 nL/min for 6 minutes into both hemispheres. Subsequent to both injections, incisions were closed via Vicryl sutures (Ethicon Inc., a Johnson & Johnson company, Somerville, NJ), a 5% lidocaine topical ointment (Fougera Pharmaceuticals Inc., a Sandoz company, Melville, NY) was applied, and the mice were placed into a heated recovery chamber to recuperate.
Primary Antibodies
The following antibodies and their respective dilutions were used to IHC stain coronal sections of 15- and 21-month-old 3xTg-AD mice: anti-Aβ 1-42 clone 12F4 reactive to the C-terminus of β-amyloid specific for the isoform ending at amino acid 42 (dilution 1:1000; Signet Laboratories, Berkeley, CA); microglia/macrophage-specific cell marker [anti-ionized calcium-binding adaptor molecule (Iba-1)], rabbit polyclonal (dilution 1:750; Wako Chemicals USA Inc., Richmond, VA); astrocyte-specific cell marker [polyclonal rabbit anti-glial fibrillary acidic protein (GFAP)] (dilution 1:1000; Dako North America, Carpinteria, CA); PHF-1 recognizing singly or doubly phosphorylated Tau at Ser396 and/or Ser404 residues (dilution 1:30; a gift from Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY); rabbit anti-GFP (dilution 1:750; Life Technologies); monoclonal anti-NeuN, clone A60, biotin conjugated (dilution 1:300; Chemicon, Billerica, MA); and monoclonal anti-GFAP–Cy3, conjugated (dilution 1:1000; Sigma-Aldrich, St. Louis, MO).
IHC Analyses
Cryoprotectant was removed from brain sections by washing with 0.15 mol/L phosphate buffer, and endogenous peroxidase activity was quenched with 3% H2O2 in 0.15 mol/L phosphate buffer. For Aβ peptide–specific stains, epitope retrieval was performed using 70% formic acid for 15 minutes. Diaminobenzidine IHC analysis was performed as previously described.27 Images from the CA1 and subiculum subregions of the hippocampal formation between bregma positions −4.20 mm and −1.82 mm were acquired under ×20 magnification, and staining intensities were quantified in a blinded manner above a preset threshold using the MCID Elite, version 6.0, imaging software program (InterFocus Imaging, a subsidiary of GE Healthcare, Cambridge, England). The average number of pixels per unit area from at least four sections per slide per mouse were analyzed (4 to 6 images for the subiculum and 16 to 20 images for the CA1 region), and the means were statistically assessed (n = 6 per injection condition). Representative light microscopic images at ×10 magnification were acquired for each immunohistologic experiment using a Zeiss Axioplan IIi microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments, Burroughs, MI).
Fluorescence Co-IHC Analysis
Coronal brain sections from 15-month-old 3xTg-AD mice receiving bilateral hippocampal injections with the rAAV2-siScr vector were processed for fluorescence co-IHC analysis to demonstrate neuronal targeting of serotype 2 viral capsids. Tissue sections were washed in PBS to remove cryoprotectant and were permeabilized for 5 minutes with PBS + 0.4% Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany), followed by blocking with PBS + 0.4% Triton X-100 + 10% normal goat serum for 1 hour. Three separate co-IHC analyses were performed using rabbit anti-GFP and monoclonal anti-NeuN, rabbit anti-GFP and rabbit polyclonal anti–Iba-1, or rabbit anti-GFP and monoclonal anti-GFAP–Cy3 antibodies diluted in PBS + 0.4% Triton X-100 + 1% normal goat serum (refer to Primary Antibodies for the specific dilutions used) and incubated overnight at 4°C. Subsequently, sections were washed 3 × 10 minutes in PBS + 0.4% Triton X-100 + 1% normal goat serum and were incubated for 2 hours with goat anti-rabbit Alexa Fluor 488 secondary antibody to detect anti-GFP and goat anti-mouse or goat anti-rabbit Alexa Fluor 568 (dilution 1:1000; Life Technologies), secondary antibody to detect anti-NeuN or anti–Iba-1, respectively, and then were washed in PBS 3 × 10 minutes. Sections were mounted and coverslipped with Mowiol medium (Sigma-Aldrich). Images were captured with equal exposure times at 20× using an AttoArc 2 mercury lamp (Carl Zeiss), an Axioplan IIi microscope equipped with a SensiCam QE camera (The Cooke Corp., Auburn Hills, MI), and SlideBook 5.0 imaging software version 5.0.08 on Windows XP (Intelligent Imaging Innovations Inc., Denver, CO).
Enzyme-Linked Immunosorbent Assay
Hippocampal tissue was microdissected from transduced 15- and 21-month-old 3xTg-AD mice, followed by storage at −80°C. Frozen tissue was weighed, homogenized, and fractionated by ultracentrifugation. Hippocampal tissue homogenates were assessed for levels of insoluble Aβ40 and Aβ42, as previously described.26 A portion of the soluble tissue fraction was used to analyze mouse TNF-α protein levels using a ready-to-use enzyme-linked immunosorbent assay (ELISA) kit (Life Technologies). Samples were diluted 1:5, and the assay was run per the manufacturer's protocol; total protein was assayed by OD measurement at 450 nm.
Imaging and Image Processing
Photomicrographs were processed consistently where brightness and contrast were applied identically over all the images in an experimental data set using Photoshop CS3 version 10.0.1 (Adobe Systems Inc., San Jose, CA). No other image-processing manipulations were performed except for photomicrographs containing digitally magnified inset images to highlight cellular morphology.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism version 5 software (GraphPad Software Inc., San Diego, CA) by means of one-way analysis of variance followed by Bonferroni's posttest. Figure legends and results sections specify each statistical test used and associated P values for each experiment.
Results
rAAV2-Mediates RNA Interference Knockdown of TNF-RI and TNF-RII in Vitro
Three rAAV2 vectors (Figure 1A) were designed to express both eGFP under the regulatory control of the CMV immediate-early promoter, allowing for visualization of vector-transduced cells, and a 7SK RNA polymerase III promoter–driven siRNA.28 Before rAAV2 packaging, the pFBGR shuttle vectors harboring siTNF-RI, siTNF-RII, or siScr were tested in vitro via transient cotransfection of BHK cells with respective TNF-RI or TNF-RII cDNA-encoding plasmids (Figure 1, B–Q). Forty-eight hours after transfection, BHK cells were processed and evaluated for eGFP and TNF-RI or TNF-RII expression by fluorescence co-IHC analysis. We observed a marked reduction in TNF-RI expression (mean ± SD: 78.9% ± 1.520%) in BHK cells cotransfected with siTNF-RI and TNF-RI plasmids (Figure 1, H and I), whereas BHK cells receiving siScr control plasmid and TNF-RI plasmid retained TNF-RI expression (Figure 1, D and E). Similarly, cells cotransfected with siTNF-RII and TNF-RII plasmids demonstrated a substantial reduction in TNF-RII expression (mean ± SD: 78.8% ± 2.115%) (Figure 1, P and Q) compared with cells transfected with siScr plasmid (Figure 1, L and M). These data indicated that the constructed rAAV2 vectors were selective and effective at knocking down TNF-RI or TNF-RII expression in vitro and that they were subsequently packaged into rAAV2 capsids.
rAAV2-Expressing siRNA Vectors Selectively Target Pyramidal Neurons of the CA1 Sublayer in Vivo
We next aimed to determine whether the functional siRNA-expressing viral plasmids packaged into rAAV2 virions were neuronally selective when delivered stereotactically to the mouse brain. To this end, 2- and 12 month-old 3xTg-AD mice, which develop AD-related amyloid and Tau pathologies,10,11 received stereotactic bilateral hippocampal injections of rAAV2-siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII and were aged to 15 and 21 months, respectively (Figure 2A). To ensure that in vivo rAAV2 transduction selectively targeted the neuronal cell population, we fluorescently colabeled coronally sectioned 15-month-old 3xTg-AD brain tissue transduced with rAAV2-siScr using anti-eGFP plus anti-NeuN, anti-GFAP, or anti–Iba-1 primary antibodies and performed immunofluorescence microscopy to localize eGFP-positive cells (Figure 2, B–J). On microscopic visualization, it was demonstrated that eGFP expression specifically colocalized with NeuN-positive cells (Figure 2, B–D), hence confirming that the rAAV2 vectors were selectively transducing neurons and expressing the eGFP reporter gene product in vivo. Neither astrocytes nor microglial cells were transduced as assessed by colabeling for GFP and anti-GFAP (Figure 2, E–G) or anti–Iba-1 (Figure 2, H–J), respectively.
Figure 2.
rAAV2-expressing siRNA vectors selectively targeted pyramidal neurons of the CA1 sublayer. A: The schematic diagram illustrates the experimental design of 3xTg-AD mice receiving stereotactic bilateral hippocampal infusions of rAAV2 at 2 or 12 months and harvested at 15 or 21 months, respectively. Coronally sectioned 15-month-old rAAV2-siScr–injected tissue was co-immunostained with an anti-eGFP primary antibody in conjunction with anti-NeuN (neurons) (B–D), anti-GFAP (astrocytes) (E–G), or anti–Iba-1 (microglia) (H–J) to localize eGFP-positive cells. Boxed areas are shown as insets at a higher magnification. Scale bar = 500 μm.
In Vivo rAAV2 Viral Vector–Mediated Knockdown of Neuronal TNF-RII in 3xTg-AD Mice Leads to a Significant Elevation in Extracellular Aβ42 Plaque Deposition at Later Stages of Disease
An accruing body of evidence suggests an intimate association between TNF-α receptor signaling and Aβ accumulation in the setting of AD.6–8,29 To investigate the cell- and stage-specific effect of neuronal knockdown of TNF-RI and/or TNF-RII on Aβ pathology, IHC analysis was performed. Coronal brain sections of age-matched 15- and 21-month-old mice bilaterally transduced with rAAV2-siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII at 2 and 12 months, respectively, were immunostained with the anti-12F4 antibody, which specifically detects extracellular Aβ42 deposits (Figure 3, A–H). Deposition in the hippocampal CA1 and subicular regions was subsequently assessed by quantitative image analysis (Figure 3, I–L). Representative hippocampal images at ×10 magnification are displayed for 3xTg-AD mice injected with rAAV2 at 2 months old (Figure 3, A–D) and 12 months old (Figure 3, E–H). On image analysis, it was revealed that in earlier stages of disease, when neuronal TNF-R expression was suppressed starting at 2 months of age, extracellular plaque deposition did not significantly change in mice receiving rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII compared with that of age-matched rAAV2-siScr–injected 3xTg-AD mice (Figure 3, I and J). However, knocking down TNF-RII starting at 12 months of age, which represents an age when 3xTg-AD mice harbor appreciable AD-associated pathology,27 leads to a striking and significant increase in 12F4-positive plaque deposition in the CA1 and subicular subregions of the hippocampus relative to TNF-RI, TNF-RI+RII, or control vector knockdown cohorts (Figure 3, K and L).
Figure 3.
In vivo rAAV2 viral vector–mediated knockdown of neuronal TNF-RII in 3xTg-AD mice led to a significant elevation of extracellular Aβ42 plaque deposition at later stages of disease. Coronal brain sections from 15-month-old (A–D) and 21-month-old (E–H) 3xTg-AD mice that received bilateral hippocampal injections at 2 and 12 months of age, respectively, with rAAV2-siScr (A and E), rAAV2–siTNF-RI (B and F), rAAV2–siTNF-RII (C and G), or rAAV2–siTNF-RI+RII (D and H) were IHC analyzed for extracellular Aβ42 plaque deposition using a human Aβ42-specific antibody, 12F4, and were subjected to diaminobenzidine IHC analysis. Representative images of the hippocampus were captured at ×10 magnification (A–H). Scale bar = 500 μm. The staining intensities in the CA1 (I and K) and subiculum (J and L) subregions for extracellular Aβ42-postive plaques were elucidated. Statistical analyses were performed via one-way analysis of variance and Bonferroni’s posttest. Error bars represent SEM. n = 6. ∗P < 0.0001.
To further assess Aβ pathology, we measured levels of soluble and insoluble Aβ40 and Aβ42 peptide levels in the brains of 3xTg-AD mice injected at 2 and 12 months by ELISA (Figure 4). We observed a significant elevation in insoluble Aβ42 levels in mice receiving neuronal rAAV2–siTNF-RII at 2 (Figure 4F) and 12 (Figure 4H) months of age and increased insoluble Aβ40 levels at 12 months of age (Figure 4G) compared with the rAAV2-siScr, rAAV2–siTNF-RI, and rAAV2–siTNF-RI+RII cohorts. No significant differences were discernible for the soluble Aβ40 and Aβ42 fractions in transduced 2- and 12-month-old 3xTg-AD mice. Taken together, these data indicate that suppressing neuronal TNF-RII–mediated signaling in the 3xTg-AD mouse at later stages of disease leads to marked Aβ plaque pathology.
Figure 4.
Neuronal rAAV2–siTNF-RII delivery to 3xTg-AD mice injected at 12 months old led to a significant increase in insoluble Aβ40 and Aβ42 levels. Hippocampal tissue homogenates of 3xTg-AD mice injected at 2 months old (A, B, E, and F) and 12 months old (C, D, G, and H) were subjected to soluble (A–D) and insoluble (E–H) Aβ40 (A, C, E, and G) and Aβ42 (B, D, F, and H) ELISA. One-way analysis of variance and Bonferroni’s posttest were performed. Error bars denote SEM. n = 3 to 4. ∗P < 0.05, ∗∗P < 0.001, and ∗∗∗P < 0.0001.
TNF-RII Knockdown in Neurons Via rAAV2 Viral Vector Delivery at 12 Months of Age in 3xTg-AD Mice Robustly Increases PHF Formation Compared with TNF-RI, TNF-RI+RII, or Control Vector Knockdown
To examine the consequence of neuronal TNF-R knockdown in 3xTg-AD mice on Tau-related pathology, we assessed intracellular NFTs. Because we observed a dramatic effect on extracellular Aβ deposition in the later stages of disease, and pathologic Aβ peptides may directly or indirectly enhance the formation of NFTs,30–32 we assessed PHF pathology. Using the PHF-1 antibody, which recognizes singly or doubly phosphorylated Ser396 and Ser404 epitopes, we immunostained coronal brain sections of 21-month-old 3xTg-AD mice transduced with rAAV2siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII (Figure 5, A–D). Quantitative image analysis demonstrated that rAAV2–siTNF-RII delivery led to robust enhancement of PHF-1–immunopositive staining intensity that was absent in mice transduced with rAAV2-siScr, rAAV2–siTNF-RI, and rAAV–siTNF-RI+RII (Figure 5E), indicating that the suppression of only TNF-RII, but not TNF-RI or TNF-RI+RII, affects the severity of amyloid (Figures 3, E–H, J, and K, and 4, G and H) and NFT pathologies in the later stages of disease.
Figure 5.
TNF-RII knockdown in neurons via AAV2 viral vector delivery at 12 months of age in 3xTg-AD mice robustly increased PHF formation compared with TNF-RI, TNF-RI+RII, or control vector knockdown. A–D: Coronal brain sections of 3xTg-AD mice bilaterally and hippocampally transduced at 12 months of age were harvested at 21 months and subjected to diaminobenzidine IHC analysis for PHF Tau (Ser396 and Ser404) using the PHF-1 antibody. Representative ×10 images are provided. Insets are digitally enhanced ×3 images of the designated immunostained CA1 region to highlight cellular morphology. Scale bar = 500 μm. E: Staining intensities of PHF-1 immunoreactive cells were quantitated. One-way analysis of variance and Bonferroni’s posttest were performed. Error bars represent SEM. n = 6. ∗P < 0.0001.
Selective Neuronal TNF-RII or TNF-RI+RII Knockdown Via AAV2-siRNA Transduction Leads to an Unexpected Reduction in Iba-1–Positive Microglial Staining Intensities
Microglia and astrocytes are highly plastic cells that interact and react to changes in the central nervous system microenvironment.33,34 Astrocytes are critical in brain homeostasis and are metabolically coupled in many processes with neurons, whereas microglia survey the brain parenchyma to detect physiologic disturbances. Microglial activation is partly controlled by neurons that produce or express a large number of regulatory factors, and, often, loss of one or more of these ligands results in the activation of microglia.35–38 Given the importance of neuron-microglia and neuron-astrocyte interactions, we investigated the extrinsic role of neuronal TNF-R signaling on microglia and astrocytes in the setting of experimental AD. Coronal sections from 15- and 21-month-old 3xTg-AD mice that were bilaterally injected at 2 and 12 months of age, respectively, with rAAV2-siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII were IHC stained with the Iba-1 microglia/macrophage-specific marker (Figure 6) or the GFAP (data not shown) to assess whether modulations in neuronal TNF-R signaling alter microglial or astrocyte marker expression. Unexpectedly, the staining intensities of Iba-1–positive microglia in 15- and 21-month-old 3xTg-AD mice were significantly reduced when TNF-RII or TNF-RI+RII expression was suppressed (Figure 6, C, D, G–J). Knockdown of neuronal TNF-RI led to a significant enhancement of Iba-1–positive microglia at 15 months of age compared with age-matched control vector–injected 3xTg-AD mice (Figure 6I). The difference in Iba-1 staining intensities between 15-month-old 3xTg-AD mice transduced with rAAV2–siTNF-RI or rAAV2-siScr was not observed in the cohort of mice transduced at a later stage of disease (Figure 6J). The staining intensities of GFAP-expressing astrocytes remained unaltered for all transduction conditions (data not shown). In aggregate, these data suggest that pathways regulated by neuronal TNF-RII may be important in providing activation signals to local microglia as AD-related pathology develops.
Figure 6.
Selective neuronal TNF-RII or TNF-RI+RII knockdown via rAAV2-siRNA transduction led to an unexpected reduction in Iba-1–positive microglial staining. Two- and twelve-month-old 3xTg-AD mice received bilateral hippocampal injections of rAAV2-siScr, rAAV2–siTNF-RI, rAAV2–siTNF-RII, or rAAV2–siTNF-RI+RII and were euthanized at 15 and 21 months of age, respectively. Coronal histologic sections were stained with Iba-1 microglial-specific marker, followed by diaminobenzidine IHC analysis. Representative images at ×10 magnification are shown (A–H), and insets are digitally enhanced ×4 images to demonstrate cellular morphology. Scale bar = 500 μm. Iba-1–positive pixels were enumerated in the CA1 region of the hippocampus for mice receiving viral vectors at 2 and 12 months of age (I and J). Statistical analyses performed included one-way analysis of variance and Bonferroni’s posttest. Error bars indicate SEM. n = 6. ∗P < 0.05, ∗∗P < 0.001, and ∗∗∗P < 0.0001.
Neuronal TNF-R Modulation via siRNA Technology Results in a Stage-Dependent Alteration of Endogenous TNF-α Levels
An important consideration in this study is whether modulating the expression of TNF-RI and TNF-RII affects endogenous TNF-α levels. To that end, we performed a mouse TNF-α sandwich ELISA using hippocampal protein tissue homogenates from 15- and 21-month-old 3xTg-AD mice transduced with the viral vector siRNA constructs at 2 and 12 months, respectively. In the 15-month-old cohort (transduced at 2 months of age), we observed a significant increase in TNF-α levels in mice receiving rAAV2–siTNF-RI+RII relative to the rAAV2-siScr control, suggesting that when both targets of TNF-α are dampened, there is an accumulation of soluble TNF-α (Figure 7A). TNF-R modulation initiated during ongoing disease results in a different TNF-α profile. In the 21-month-old cohort (transduced at 12 months), there is a significant reduction in TNF-α protein expression when TNF-RII or TNF-RI+RII is silenced in neurons (Figure 7B). These observations indicate that neuronal TNF-RII expression exerts differential effects on total TNF-α protein levels in a temporal-dependent manner.
Figure 7.

Neuronal TNF-R modulation resulted in a stage-dependent alteration of endogenous TNF-α levels. Hippocampal protein homogenates of 15-month-old (A) and 21-month-old (B) 3xTg-AD mice receiving bilateral intrahippocampal injections at 2 and 12 months, respectively, were subjected to TNF-α ELISA. One-way analysis of variance and Bonferroni’s posttest were performed. Error bars indicate SEM. n = 4. ∗P < 0.05, ∗∗P < 0.001, and ∗∗∗P < 0.0001.
Discussion
Studies have implicated long-term TNF-α up-regulation in the progression of AD, but the purported function of this immune modulator in disease remains unclear and intensely debated. Given the pleiotropic nature of TNF-α, we hypothesized that cell type-specific signaling at certain stages of AD pathogenesis may differentially affect hallmark amyloid and Tau pathology and accompanying neuroinflammation. We previously demonstrated that long-term nonselective abrogation of TNF-R expression in 3xTg-AD mice results in significant increases in AD-related pathology.24 In addition, long-term expression of TNF-α by rAAV2 delivery in 3xTg-AD mice results in neuronal cell loss, signifying that TNF-α is a potential contributor to neuronal death in AD.13 To this end, age and disease stage may alter neuronal TNF-R function, as demonstrated by Patel and Brewer,39 who reported that in the presence of Aβ42, aging affects neuronal survival by changing TNF-R expression. For example, neurons derived from “older” mice were more vulnerable to Aβ42 toxicity and were unable to up-regulate TNF-RI and TNF-RII surface expression compared with “middle-aged” neurons.39 Together, these data suggest that microglial TNF-R signaling is likely required at certain stages of disease to suppress pathology, whereas other brain-resident cell populations, such as neurons, may be susceptible to Aβ-induced toxicity with intact TNF-R signaling. Therefore, to selectively study the contribution of neuronal TNF-R signaling in AD, we generated rAAV2-siRNA vectors to knock down receptor expression in the early and late stages of disease. The present results demonstrate that the designed TNF-R siRNA DNA constructs are functional in knocking down their assigned receptors in vitro and subsequent to rAAV2 packaging, capable of targeting neurons in the CA1 sublayer. Sustained inhibition of TNF-RII selectively in hippocampal neurons increases the amyloid burden, elevates Tau hyperphosphorylation, and diminishes Iba-1–positive staining intensities of microglia during the later stages of disease in 3xTg-AD mice.
The downstream signaling cascades assigned to TNF-RI and TNF-RII are quite distinct, and execution of TNF-R signaling depends on a variety of conditions, including the cellular context, receptor conformation, and inflammatory milieu. Several groups have elucidated divergent signaling pathways for TNF-RI and TNF-RII in neurons. Yang et al40 reported that hippocampal neuron survival is unaffected in culture in the absence of TNF-RI; however, neurons devoid of TNF-RII are highly vulnerable to TNF-α at concentrations as low as 100 pmol/L. Furthermore, ligation of TNF-RI and TNF-RII with TNF-α induces different cellular pathways where a classical NF-κB signaling cascade is favored by TNF-RI engagement, whereas TNF-RII up-regulates p38 mitogen-activated protein kinase activity, suggesting that altered TNF-R expression affects neuronal survival and is likely a consequence of unique downstream signaling pathways.40 Moreover, TNF-RII stimulation induces the activation of alternative or noncanonical NFκB, which has been shown to down-regulate proinflammatory cytokine production.41,42 In the present study, we did not assess the number of hippocampal neurons in vector-transduced mice. Based on the aforementioned literature, it is possible that the number of hippocampal neurons expressing siTNF-RII would be reduced compared with siScr- or siTNF-RI–infused cohorts because TNF-RI seems to elicit prosurvival signals to neurons.
Evidence of the signaling dichotomy between TNF-RI and TNF-RII also exists in the disease setting. In human AD, TNF-RI protein and gene expression is increased compared with TNF-RII, and TNF-R activation is abnormal in AD brains where TNF-RI exhibits an increased binding affinity to TNF-α compared with nondemented control patients.17,43 In preclinical studies, TNF-RI promotes Aβ-induced neuronal death, and in the APP23 mouse model, deletion of TNF-RI reduced Aβ plaque pathology, improved spatial learning and memory, and decreased β-secretase levels compared with littermate control mice.16,44 Similarly, the present results show that silencing TNF-RII, but not TNF-RI or TNF-RI+RII in combination, results in dramatically enhanced 12F4-positive Aβ plaque burden (Figure 3, G, K, and L), insoluble Aβ40/Aβ42 levels (Figure 4, G and H), and PHF-1 pathology (Figure 5). These data suggest that unopposed neuron-specific TNF-RI signaling during the later stages of disease leads to exacerbated pathology, and TNF-RII exerts protective responses that may be required to counteract TNF-RI–driven signal transduction.
Although there is a breadth of literature supporting the development of therapies that inhibit TNF signaling in AD, most studies contend that pan anti-TNF therapeutics are optimal candidates for disease immunomodulation. Most of these studies have not evaluated the impact of long-term use of such agents or the cell- or stage-specific consequences of such approaches. For example, Shi et al45 demonstrated that transient intracerebroventricular infusion of infliximab, a monoclonal chimeric TNF-α antibody that binds soluble and transmembrane forms of TNF-α, to double-transgenic APP/PS1 mice reduced TNF-α levels, amyloid deposition, and hyperphosphorylated Tau pathology. Although these data are quite compelling, mice received the infliximab treatment only transiently for up to 14 days. More recently, a novel thalidomide-derived TNF-α–lowering agent, 3,6′-dithiothalidomide, which is more potent than thalidomide itself, penetrates the blood-brain barrier, and posttranscriptionally represses TNF-α, was administered over a 6-week period to 17-month-old 3xTg-AD mice and was shown to attenuate insoluble Aβ plaque burden and memory and learning deficits as measured by the Morris water maze task.46 This report is interesting because it examines TNF inhibition in late-stage disease but, similar to other studies, does not address the consequences of long-term and broad silencing of TNF signaling. The present findings support a conclusion that unopposed neuronal TNF-RI signaling, when TNF-RII has been selectively down-regulated, leads to a more severe course of AD pathogenesis. Hence, cell and receptor type–specific targeting of the TNF-Rs rather than broad TNF intervention in advanced stages of disease may represent a safer, albeit more complex, strategy by which to impede disease progression.
The significant reduction in Iba-1 intensities documented in mice receiving rAAV2–siTNF-RII or rAAV2-siTNF-RI+RII (Figure 6) strongly implicates a nonautonomous role for neuronal TNF-RII signaling in AD whereby neuronal TNF-RII may be indirectly affecting local microglial function and/or activity. Given that neurons are able to control microglial activity,36–38 it is plausible that unopposed TNF-RI signaling in neurons, when TNF-RII is down-regulated, leads to elaboration of signals to local microglia that impair functions such as microglial-mediated clearance of Aβ. There are a variety of surface molecules on microglia that facilitate communication of regulatory signals from neurons. For example, CD200 is constitutively expressed on the neuronal surface as a glycoprotein that directly communicates through cognate ligation with the microglial CD200 receptor, which exerts inhibitory signals to maintain microglia in a quiescent state. The inhibitory role of CD200 was demonstrated in a facial nerve transection model where macrophages/microglial cells were in higher numbers and displayed an activated phenotype in CD200-deficient mice.35 In addition, the CD47/integrin-associated protein expressed by neurons interacts with microglial SIRPα and is involved in phagocytic responses regulated by Fc gamma and complement receptors.47 One of the most studied microglial-neuronal interactions is the fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) expressed on neurons and microglia, respectively. Although the exact role of CX3CL1-CX3CR1 is not well understood, it has been shown that removing CX3CR1 in hAAP-J20 mice worsens Tau pathology and performance in passive avoidance and novel object recognition behaviors.48 TNF-α–converting enzyme (ADAM17), the enzyme involved in proteolytic release of soluble TNF-α, has been associated with CX3CL1 proteolysis from the cell surface. Because TNF-RI preferentially signals via soluble TNF-α ligation, it is possible that knocking down neuronal TNF-RII may lead to an increase in local ADAM17 concentrations to act on TNF-α and CX3CL1.49 Moreover, a variety of neurotransmitters may modulate microglial cell activity. For example, microglia express several glutamate receptors, including group II metabotropic receptors, and stimulation of these receptors has been shown to be associated with TNF-RI–mediated microglial neurotoxicity in a rat primary microglia culture paradigm.50 Therefore, it is conceivable that modulation of neuronal TNF-RI and TNF-RII receptor expression via siRNA technology may affect microglia-neuron communication and downstream microglial activation/function.
Although diminished microglial phagocytosis may underlie the enhanced Aβ pathology observed when neuronal TNF-RII expression is experimentally decreased, other not necessarily mutually exclusive molecular and cellular mechanisms may be involved. Increased Aβ peptide load may result from reduced Aβ-degrading enzyme levels and/or increased amyloid precursor protein (APP) processing in neurons. Hickman et al51 reported that Aβ-degrading enzyme levels, including insulysin, neprilysin, and matrix metallopeptidase 9, are significantly decreased in 14-month-old PSI-APP mice. This observation coincides with a significant increase in TNF-α transcript level at the same age, suggesting that the up-regulation of TNF-α compromises Aβ-degrading pathways.51 This report supports the possibility that unopposed TNF-RI signaling in neurons may lead to the down-regulation of enzymes involved in Aβ degradation. Furthermore, studies have revealed that β-secretase activity is augmented by TNF-RI as demonstrated through use of a dominant-negative soluble TNF inhibitor that primarily blocked TNF-RI transduction in 3xTg-AD mice. C-terminal APP fragments arising from β-secretase activity were significantly decreased in mice receiving this inhibitor.22 Moreover, deletion of the TNF-RI gene in APP23 mice was shown to abrogate Aβ generation by lowering β-secretase levels and activity, indicating that TNF-RI facilitates amyloidogenic cleavage of APP.16 Whether the observed increases in pathogenic Aβ peptide and deposition detected in 21-month-old 3xTg-AD mice receiving TNF-RII siRNA is a consequence of increased TNF-RI and/or β-secretase activity is presently unknown. However, the present data suggest that TNF-RI may not be directly altering APP processing in the present study because Aβ levels in TNF-RI siRNA–receiving 3xTg-AD mice were comparable with those in aged-matched siScr control counterparts (Figures 3 and 4).
In conclusion, we demonstrated that long-term knockdown of TNF-RII in hippocampal neurons via rAAV2 vector–mediated siRNA delivery enhances amyloid- and Tau-related pathologic features. The present work builds on existing data cautioning against the long-term use of pan anti–TNF-α therapies for AD and emphasizes the need for strategies that more selectively modulate TNF signaling in specific cell types and at different stages of disease. Moreover, these data provide a foundation for understanding how neuron-specific targeting of TNF-R signaling affects overall disease severity. Future studies will focus on dissecting the TNF-mediated mechanisms that lead to enhanced AD-associated pathology and on assessing the effects of selective TNF signaling suppression in other brain-resident populations, including microglia and astrocytes.
Acknowledgments
We thank Louis Lotta, Jr. (University of Rochester) for animal husbandry and care, Dr. Linda Callahan (University of Rochester) for microscopy advice, and Dr. Terry Wright (University of Rochester) for helpful experimental insights.
Footnotes
Supported by NIH grants F31-AG038063 (S.L.M.), RO1-AG030149 (M.K.O.), and R01-AG023593 and R01-AG026328 (W.J.B.).
Current address of W.J.B., Vaccinex, Inc., Rochester, NY.
Contributor Information
M. Kerry O'Banion, Email: kerry_obanion@urmc.rochester.edu.
William J. Bowers, Email: wbowers@vaccinex.com.
References
- 1.Huberman M., Shalit F., Roth-Deri I., Gutman B., Brodie C., Kott E., Sredni B. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J Neuroimmunol. 1994;52:147–152. doi: 10.1016/0165-5728(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 2.Motta M., Imbesi R., Di Rosa M., Stivala F., Malaguarnera L. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Parachikova A., Agadjanyan M.G., Cribbs D.H., Blurton-Jones M., Perreau V., Rogers J., Beach T.G., Cotman C.W. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging. 2007;28:1821–1833. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K.S., Chung J.H., Choi T.K., Suh S.Y., Oh B.H., Hong C.H. Peripheral cytokines and chemokines in Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 5.Meda L., Cassatella M.A., Szendrei G.I., Otvos L., Jr., Baron P., Villalba M., Ferrari D., Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 6.Fillit H., Ding W.H., Buee L., Kalman J., Altstiel L., Lawlor B., Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- 7.Dickson D.W. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Paganelli R., Di Iorio A., Patricelli L., Ripani F., Sparvieri E., Faricelli R., Iarlori C., Porreca E., Di Gioacchino M., Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 9.Collins J.S., Perry R.T., Watson B., Jr., Harrell L.E., Acton R.T., Blacker D., Albert M.S., Tanzi R.E., Bassett S.S., McInnis M.G., Campbell R.D., Go R.C. Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer disease: the NIMH Alzheimer Disease Genetics Initiative. Am J Med Genet. 2000;96:823–830. doi: 10.1002/1096-8628(20001204)96:6<823::aid-ajmg26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Oddo S., Caccamo A., Kitazawa M., Tseng B.P., LaFerla F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Janelsins M.C., Mastrangelo M.A., Oddo S., LaFerla F.M., Federoff H.J., Bowers W.J. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janelsins M.C., Mastrangelo M.A., Park K.M., Sudol K.L., Narrow W.C., Oddo S., LaFerla F.M., Callahan L.M., Federoff H.J., Bowers W.J. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal B.B., Samanta A., Feldmann M. TNF receptors. In: Oppenheim J.Ja.F.M., editor. Cytokine Reference. Academic Press; London: 2000. pp. 1620–1632. [Google Scholar]
- 15.Wajant H., Pfizenmaier K., Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 16.He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X., Yang L., He P., Li R., Shen Y. Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer's disease and non-demented patients. J Alzheimers Dis. 2010;19:621–630. doi: 10.3233/JAD-2010-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng B., Christakos S., Mattson M.P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 19.Barger S.W., Horster D., Furukawa K., Goodman Y., Krieglstein J., Mattson M.P. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce A.J., Boling W., Kindy M.S., Peschon J., Kraemer P.J., Carpenter M.K., Holtsberg F.W., Mattson M.P. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 21.Bruce-Keller A.J., Geddes J.W., Knapp P.E., McFall R.W., Keller J.N., Holtsberg F.W., Parthasarathy S., Steiner S.M., Mattson M.P. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J Neuroimmunol. 1999;93:53–71. doi: 10.1016/s0165-5728(98)00190-8. [DOI] [PubMed] [Google Scholar]
- 22.McAlpine F.E., Lee J.K., Harms A.S., Ruhn K.A., Blurton-Jones M., Hong J., Das P., Golde T.E., LaFerla F.M., Oddo S., Blesch A., Tansey M.G. Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobinick E., Gross H., Weinberger A., Cohen H. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. MedGenMed. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery S.L., Mastrangelo M.A., Habib D., Narrow W.C., Knowlden S.A., Wright T.W., Bowers W.J. Ablation of TNF-RI/RII expression in Alzheimer's disease mice leads to an unexpected enhancement of pathology implications for chronic pan-TNF-alpha suppressive therapeutic strategies in the brain. Am J Pathol. 2011;179:2053–2070. doi: 10.1016/j.ajpath.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 26.Ryan D.A., Mastrangelo M.A., Narrow W.C., Sullivan M.A., Federoff H.J., Bowers W.J. Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer's disease mice. Mol Ther. 2010;18:1471–1481. doi: 10.1038/mt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrangelo M.A., Bowers W.J. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer's disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koper-Emde D., Herrmann L., Sandrock B., Benecke B.J. RNA interference by small hairpin RNAs synthesised under control of the human 7S K RNA promoter. Biol Chem. 2004;385:791–794. doi: 10.1515/BC.2004.103. [DOI] [PubMed] [Google Scholar]
- 29.Buchhave P., Zetterberg H., Blennow K., Minthon L., Janciauskiene S., Hansson O. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2010;31:1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Blurton-Jones M., Laferla F.M. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J., Dickson D.W., Lin W.L., Chisholm L., Corral A., Jones G., Yen S.H., Sahara N., Skipper L., Yager D., Eckman C., Hardy J., Hutton M., McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 32.Gotz J., Chen F., van Dorpe J., Nitsch R.M. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 33.Itagaki S., McGeer P.L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 34.Serrano-Pozo A., Mielke M.L., Gomez-Isla T., Betensky R.A., Growdon J.H., Frosch M.P., Hyman B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer's disease. Am J Pathol. 2011;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoek R.M., Ruuls S.R., Murphy C.A., Wright G.J., Goddard R., Zurawski S.M., Blom B., Homola M.E., Streit W.J., Brown M.H., Barclay A.N., Sedgwick J.D. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 36.Wright G.J., Puklavec M.J., Willis A.C., Hoek R.M., Sedgwick J.D., Brown M.H., Barclay A.N. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 37.Tan J., Town T., Mullan M. CD45 inhibits CD40L-induced microglial activation via negative regulation of the Src/p44/42 MAPK pathway. J Biol Chem. 2000;275:37224–37231. doi: 10.1074/jbc.M002006200. [DOI] [PubMed] [Google Scholar]
- 38.Mott R.T., Ait-Ghezala G., Town T., Mori T., Vendrame M., Zeng J., Ehrhart J., Mullan M., Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- 39.Patel J.R., Brewer G.J. Age-related changes to tumor necrosis factor receptors affect neuron survival in the presence of beta-amyloid. J Neurosci Res. 2008;86:2303–2313. doi: 10.1002/jnr.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Lindholm K., Konishi Y., Li R., Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauert H., Wicovsky A., Muller N., Siegmund D., Spindler V., Waschke J., Kneitz C., Wajant H. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 2010;285:7394–7404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tas S.W., Vervoordeldonk M.J., Hajji N., Schuitemaker J.H., van der Sluijs K.F., May M.J., Ghosh S., Kapsenberg M.L., Tak P.P., de Jong E.C. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 43.Culpan D., MacGowan S.H., Ford J.M., Nicoll J.A., Griffin W.S., Dewar D., Cairns N.J., Hughes A., Kehoe P.G., Wilcock G.K. Tumour necrosis factor-alpha gene polymorphisms and Alzheimer's disease. Neurosci Lett. 2003;350:61–65. doi: 10.1016/s0304-3940(03)00854-1. [DOI] [PubMed] [Google Scholar]
- 44.Li R., Yang L., Lindholm K., Konishi Y., Yue X., Hampel H., Zhang D., Shen Y. Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. J Neurosci. 2004;24:1760–1771. doi: 10.1523/JNEUROSCI.4580-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J.Q., Shen W., Chen J., Wang B.R., Zhong L.L., Zhu Y.W., Zhu H.Q., Zhang Q.Q., Zhang Y.D., Xu J. Anti-TNF-alpha reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res. 2011;1368:239–247. doi: 10.1016/j.brainres.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 46.Tweedie D., Ferguson R.A., Fishman K., Frankola K.A., Van Praag H., Holloway H.W., Luo W., Li Y., Caracciolo L., Russo I., Barlati S., Ray B., Lahiri D.K., Bosetti F., Greig N.H., Rosi S. Tumor necrosis factor-alpha synthesis inhibitor 3,6′-dithiothalidomide attenuates markers of inflammation: Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J Neuroinflammation. 2012;9:106. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldenborg P.A., Gresham H.D., Lindberg F.P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho S.H., Sun B., Zhou Y., Kauppinen T.M., Halabisky B., Wes P., Ransohoff R.M., Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garton K.J., Gough P.J., Blobel C.P., Murphy G., Greaves D.R., Dempsey P.J., Raines E.W. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 50.Taylor D.L., Jones F., Kubota E.S., Pocock J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25:2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]