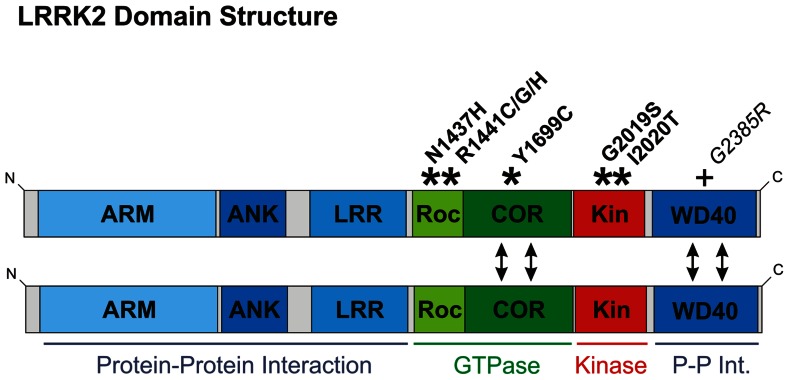
FIGURE 1.
Domain structure of LRRK2. Domains are color-coded according to function: those implicated in protein–protein interaction are depicted in blue; domains involved in GTPase function are green; and the kinase domain red. Here, LRRK2 is depicted as a dimer, although LRRK2 also exists as monomers and in higher molecular weight complexes (Greggio et al., 2008). Dimerization is likely to be mediated by the COR and/or WD40 domains (double-headed arrows). COR domains are established as dimerization devices in ROCO proteins (Gotthardt et al., 2008), whilst ablation of the WD40 domain has been reported to disrupt LRRK2 dimerization (Jorgensen et al., 2009). The location of pathological mutations proven to segregate with Parkinson’s disease are shown with asterisks and bold font. Although only considered a risk factor, the G2385R mutation is also depicted with a plus sign, since this mutation is mentioned in the main text and is very frequent amongst Asian populations. ARM, armadillo repeat; ANK, ankyrin repeat; LRR, leucine-rich repeat; Roc, ras of complex proteins; COR, c-terminal of Roc; Kin, kinase.