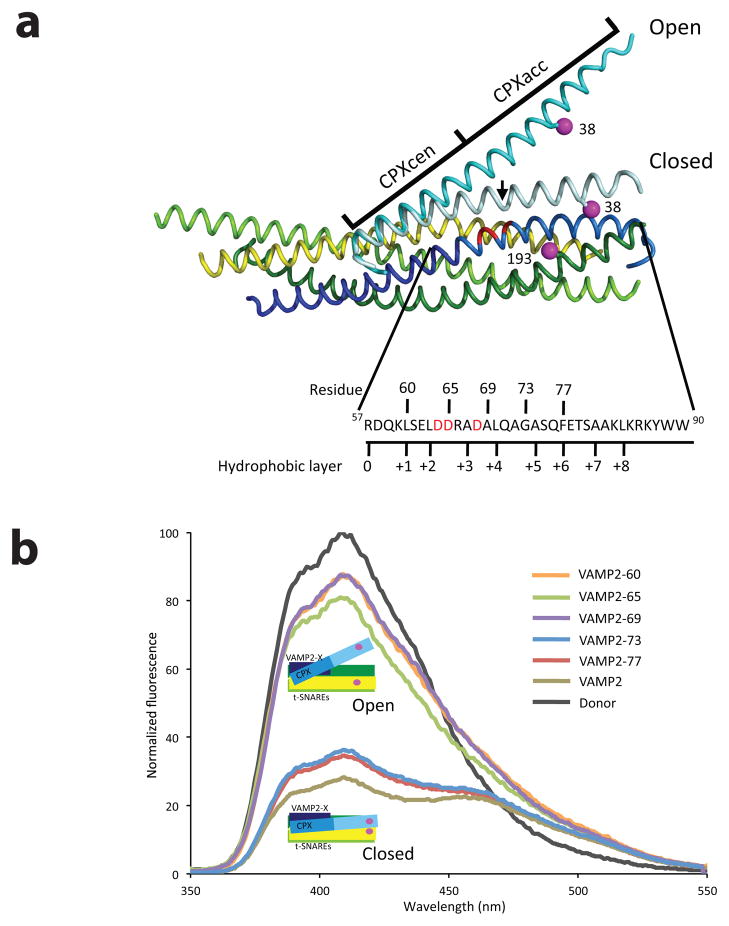
Figure 1.
Zippering of one turn of VAMP2 helix triggers the switch in CPX position (a) Superposition of the structures of the pre- and post-fusion CPX-SNARE complexes20,24, showing the FRET label positions. Syntaxin1 (residues 190–250) is in yellow, SNAP 25 N-terminal SNARE motif (residues 10–74) is in lime, SNAP 25 C-terminal SNARE motif (residues 141–203) is in green and VAMP2 is in blue (residues 25–60 dark blue; residues 61–96 in light blue). The “switch” residues (Asp 64, Asp 65 and Asp 68) are marked in red. The Complexin (residues 26–73) in the pre-fusion complex is in cyan, while in the post-fusion complex is in light cyan. The FRET label positions, residue 193 on SNAP25 and 38 on Complexin, are marked in magenta. The sequence of the C-terminal hydrophobic layer of VAMP2 (residues 57–90) with the C-terminal truncations tested in this paper (denoted by the residue number) is also shown. The black arrow in the post-fusion structure references CPX residue 48, the demarcation line between CPXcen and CPXacc. (b) FRET experiments with C-terminal truncations of VAMP2. Fluorescence emission spectra of Stilbene and Bimane labeled CPX-SNARE complexes containing VAMP2-60 (residues 25–60, orange), VAMP2-65 (residues 25–65, green), VAMP2-69 (residues 25–69, purple), VAMP2-73 (residues 25–73, blue), VAMP2-77 (residues 25–77, red), and VAMP2 (residues 1–96, olive). A representative emission spectrum of a Stilbene (Donor)-only CPX-SNARE complex is shown in black. The donor-only spectrum was identical in all CPX-SNARE complexes