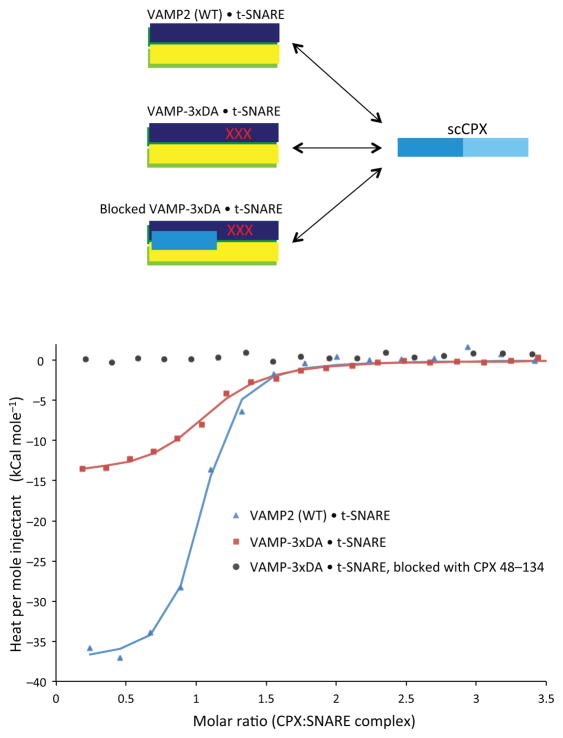
Figure 3.
Interaction of CPXcen with Asp residues 64, 65 and 68 on VAMP2 provides thermodynamic driving force for the switch. Calorimetric titrations of superclamp CPX (scCPX; residues 1–134 with D27L, E34F, R37A mutations) into assembled SNARE complexes containing t-SNAREs and either wild-type (WT) VAMP2 (blue triangles), VAMP2-3xDA (red squares), or VAMP-3xDA with the CPXcen binding site blocked by CPX 48–134 (black circles). The solid lines represent the best fit to the corresponding data points using a nonlinear least squares fit with a one-set-of-sites model. The results of the fits are given in Table 2. All experiments were performed in triplicate at 37°C, and a representative thermogram is shown.