Abstract
Background:
We seek to determine if testosterone levels below the accepted castration threshold (50 ng/dL) have an impact on time to progression to castrate-resistant prostate cancer (CRPC).
Methods:
This is a prospective cohort series of patients undergoing androgen deprivation therapy (ADT) with luteinizing hormone-releasing hormone agonist or antagonist at a tertiary centre from 2006 to 2011. Serum testosterone level was assessed every 3 months. Patients with any testosterone >50 ng/dL were excluded. Patients were stratified into groups based on those achieving mean testosterone levels <20 ng/dL and <32 ng/dL. Progression to CRPC was assessed with the Kaplan-Meier method and compared with the log-rank test.
Results:
A total of 32 patients were included in this study. Mean patient follow-up was 25.7 months. Patients with a 9-month serum testosterone <32 ng/dL had a significantly increased time to CRPC compared to patients with testosterone 32 to 50 ng/dL (p = 0.001, median progression-free survival (PFS) 33.1 months [<32 ng/dL] vs. 12.5 months [>32 ng/dL]). Patients with first year mean testosterone <32 ng/dL also had a significantly increased time to CRPC compared to 32 to 50 ng/dL (p = 0.05, median PFS 33.1 months [<32 ng/dL] vs. 12.5 months [32–50 ng/dL]). A testosterone <20 ng/dL compared to 20 to 50 ng/dL did not significantly predict with time to CRPC.
Conclusion:
This study supports a lower testosterone threshold to define optimal medical castration (T <32 ng/dL) than the previously accepted standard of 50 ng/dL. Testosterone levels during ADT serve as an early predictor of disease progression and thus should be measured in conjunction with prostate-specific antigen.
Introduction
Androgen deprivation therapy (ADT) has been a cornerstone in the treatment of advanced prostate cancer since its establishment by Huggins and Hodges in 1941.1 Prostatic adenocarcinoma undergoes a reduction in gland size and an increase in interglandular connective tissue during ADT.2,3 Although residual tumour remains3 and there is an inevitable progression to castrate-resistant prostate cancer (CRPC), marked symptom reduction is experienced on initiation of ADT.4 ADT was classically accomplished surgically by bilateral orchiectomy. Several studies have demonstrated that testosterone levels are usually 20 ng/dL or lower after surgical castration.5,6
The discovery of luteinizing hormone-releasing hormone (LHRH) made available a novel approach to medical castration through suppression of the hypothalamic-pituitary-gonadal axis without the thromboembolic effects of estrogens.7 Medical ADT is often favoured over orchiectomy because of the potential for intermittent androgen deprivation, lack of procedural complications and possible psychological benefits.
Before 1995, testosterone was measured with double-isotope-derivative dilution techniques which were not accurate below 50 ng/dL.8 Consequently, <50 ng/dL traditionally defined adequate medical castration.6,9 The advent of a chemiluminescent assay in 1995 has significantly improved the accuracy of testosterone measurements in the castrate range (<50 ng/dL),10 allowing further study of the clinical implications of testosterone levels in this range.6,11,12
Despite the widespread use of ADT, a significant proportion of patients undergoing ADT do not achieve testosterone levels equivalent to surgical castration. In a recent review, up to 12.5% did not achieve levels below 50 ng/dL and up to 37.5% did not achieve levels below 20 ng/dL.13 Clinical practice guidelines are vague on evaluating and managing supra-castrate testosterone levels. The latest guidelines from the National Comprehensive Cancer Network suggest further hormonal manipulation if testosterone levels exceed 50 ng/dL, but make no recommendations on when or if testosterone should be measured.14 While the European Association of Urology guidelines provide more guidance as to when testosterone should be measured, no direct evidence supports these recommendations.15 They suggest the measurement of serum testosterone 1 month after initiating ADT to check the testosterone nadir, as well as at 6 months to ensure castrate levels. If appropriate castration levels are not achieved, these guidelines recommend switching ADT agent or performing orchiectomy. It is also recommended that serum testosterone be assessed when progression to CRPC is suspected. However, these guidelines make no clear recommendation on an appropriate level of testosterone to define medical castration.15
To ascertain the clinical impact of serum testosterone levels on progression to CRPC, we present the outcomes of our prospective series of patients undergoing ADT by a single urologic oncologist.
Methods
After institutional review board approval, we prospectively included consecutive patients treated with ADT for prostate cancer in this cohort study. Patients were excluded if they had non-castrate testosterone levels (>50 ng/dL) at any time during follow-up or if testosterone measurements were not available at least 6 months after ADT initiation. Indications for ADT were recurrence after local therapy, locally advanced disease, metastatic disease and for concurrent treatment with primary external beam radiotherapy for D’Amico intermediate- or high-risk disease. Patients were treated by a single physician (BS) at a tertiary centre (McMaster University, Hamilton, Ontario, Canada) from 2006 to 2011.
LHRH agonist (goserelin, leuprolide or triptorelin) or antagonist (degarelix) were administered at recommended intervals and doses were based on the product monographs. For LHRH agonists, 3-month depots were used and a 1-month course of bicalutamide was administered on ADT initiation for testosterone flare protection. For LHRH antagonists, 1-month depots were used with no additional anti-androgen.
After initiation of ADT, serum prostate-specific antigen (PSA) and total testosterone were routinely measured every 3 months for the duration of the study. Serum testosterone was measured using at a commercial laboratory with a competitive chemiluminescent immunoassay (ADVIA Centaur Testosterone Assay, Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Reportable ranges of this assay are from 10 ng/dL (0.35 nmol/L) to 7500 ng/dL (260.0 nmol/L).16 At a testosterone concentration of 48.6 ng/dL (1.7 nmol/L) the within-assay coefficient of variance was 11.3%.16 Serial testosterone measurements were performed up to a week before each ADT agent administration. Patients were considered to have progressed to CRPC after 2 consecutive rises in PSA above nadir, clinical progression, or death from disease. All CRPC patients were referred for evaluation of their eligibility for chemotherapy.
To analyze the effect of testosterone levels on progression to CRPC, patients were stratified into risk groups based on 6-month and 9-month absolute testosterone levels, as well as mean testosterone levels among all measurements in the first 12 months following initiation of ADT. At each of these time points, patients were stratified into 2 groups: (1) those below the specified testosterone threshold and (2) those above the specific threshold. Testosterone thresholds of 20 ng/dL and 32 ng/dL were used. Time points and the castration threshold of 20 ng/dL (surgical castration equivalent) were chosen a priori. Post-hoc analysis was performed with a threshold of 32 ng/dL based on recent evidence that testosterone levels above this threshold are associated with a decreased time to CRPC.12
Baseline characteristics collected at the time of diagnosis included age, tumour grade, previous local treatment, PSA, tumour stage and presence of metastases. Baseline characteristics were compared between risk groups using the Student’s t-test and chi-squared test. Probability of progression to CRPC was assessed using the Kaplan-Meier method and compared using the log-rank test. Two-tailed p-values less than 0.05 were considered significant. Statistical analysis was performed with SPSS statistics version 17.0 (IBM Software, Armonk, NY).
Results
Of the 39 patients receiving ADT prospectively followed, 32 patients met inclusion criteria for this study. Seven patients were excluded because testosterone levels for at least 6 months were not available or interpretable due to patient death, care transfer or ceasing ADT.
We tallied patients’ baseline characteristics (Table 1). Mean PSA at diagnosis was 70.8 ng/mL and 19 patients had high-grade cancers (Gleason 8–10) at diagnosis. Thirteen patients had undergone radical prostatectomy at some point in their care and 8 patients underwent radiotherapy. Only 3 patients initially presented with locally advanced disease (stage T3 or greater) and 14 patients had clinical evidence of metastases at diagnoses. Mean patient follow-up was 25.7 months with 50.0% of all patients free of CRPC at last follow-up. Four deaths occurred due to CRPC, of whom 3 patients received chemotherapy prior to death.
Table 1.
Baseline patient characteristics of all patients, stratified by mean 12-month T threshold of 32 ng/dL
| Characteristic | All with T | T ≤32 ng/dL (1-year average) | T = 32–50ng/dL (1-year average)* | p value | |
|---|---|---|---|---|---|
| n | 32 | 28 | 4 | ||
| Mean T | 17.0 ng/dL | 13.7 ng/dL | 39.8 ng/dL | ||
| Age at diagnosis, years (SD) | 72.9 (8.1) | 73.1 (8.4) | 71.6 (5.6) | 0.74 | |
| Grade at diagnosis | Gleason 6, n (%) | 4 | 4 | 0 | 0.25 |
| Gleason 7, n (%) | 6 | 4 | 2 | ||
| Gleason 8–10, n (%) | 19 | 17 | 2 | ||
| Gleason unknown | 3 | 3 | 0 | ||
| Prostatectomy, n (%) | 13 | 11 | 2 | 0.68 | |
| PSA at diagnosis, ng/mL (SD) | 70.8 (124.9) | 77.4 (132.2) | 24.7 (27.3) | 0.44 | |
| Tumour stage at diagnosis, n (%) | T1, n (%) | 12 | 11 | 1 | 0.13 |
| T2, n (%) | 15 | 13 | 2 | ||
| T3, n (%) | 2 | 2 | 0 | ||
| T4 | 1 | 0 | 1 | ||
| T stage unknown | 2 | 2 | 0 | ||
| Metastases at diagnosis, n (%) | Bone metastasis | 5 | 5 | 0 | 0.36 |
| Distant + nodes | 2 | 2 | 0 | 0.84 | |
| Nodes | 7 | 6 | 1 |
T: testosterone; SD: standard deviation; PSA: prostate-specific antigen.
Patients with mean 12-month T >50 ng/dL were excluded from analyses.
Of the 32 included patients, 28 (87.5%) achieved a mean testosterone below 32 ng/dL in the first 12 months (mean: 13.73 ng/dL) and 4 did not (33.75 ng/dL, 33.75 ng/dL, 43.76 ng/dL, 47.76 ng/dL; mean: 39.75 ng/dL). Baseline characteristics did not differ significantly between these groups (Table 1).
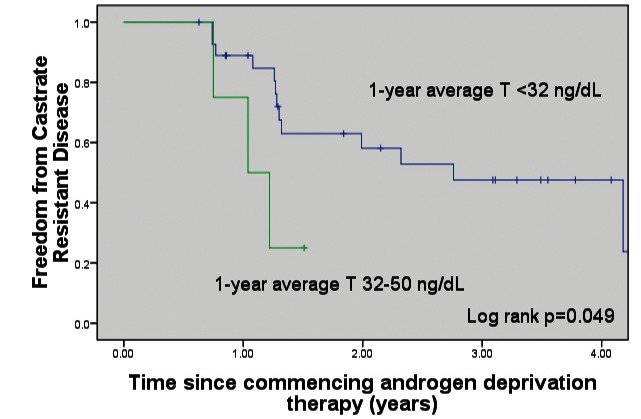
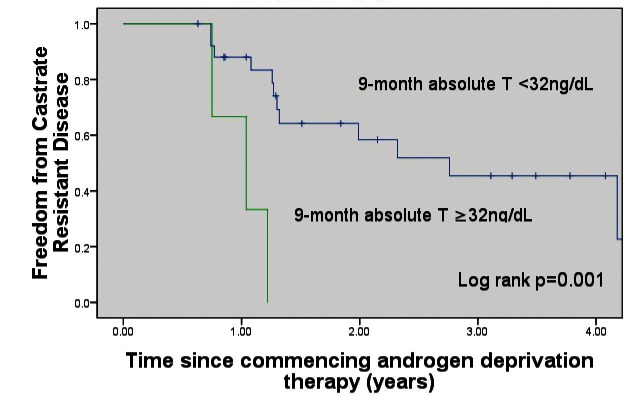
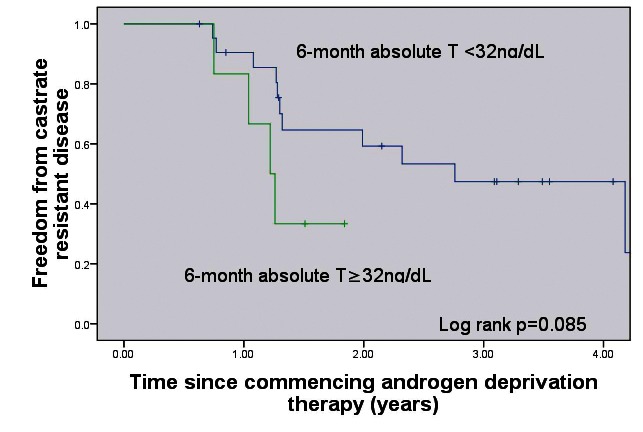
Patients with 1-year mean testosterone <32 ng/dL had a significantly increased time to CRPC (p = 0.05, median CRPC-free survival 33.1 months [<32 ng/dL] vs. 12.5 months (32–50 ng/dL) (Fig. 1). Patients with a 9-month absolute testosterone measurement <32 ng/dL had a significantly increased time to CRPC (p = 0.001, median CRPC-free survival 33.1 months [<32 ng/dL] vs. 12.5 months [>32 ng/dL]) (Fig. 2). Patients with a 6-month absolute testosterone <32 ng/dL had an increased time to CRPC, which was not statistically significant (p = 0.085, median CRPC-free survival 33.1 months [<32 ng/dL] vs. 14.6 months [>32 ng/dL]) (Fig. 3).
Fig. 1.
Freedom from castrate-resistant prostate cancer based on 1-year average testosterone levels. T: testosterone.
Fig. 2.
Freedom from castrate-resistant prostate cancer based on 9-month absolute testosterone levels. T: testosterone.
Fig. 3.
Freedom from castrate-resistant prostate cancer based on 6-month absolute testosterone levels. T: testosterone.
Of the initial 32 patients, 18 (56.3%) achieved a mean testosterone below 20 ng/dL (mean: 7.90 ng/dL) in the first 12 months, while 14 did not (mean: 28.89 ng/dL). Baseline characteristics did not differ significantly between these groups. A testosterone threshold of 20 ng/dL at 6-months, 9-months or a 1-year mean did not correlate with time to progression to CRPC (p > 0.05). Single breakthrough increases >20 ng/dL or >32 ng/dL in the first year also did not significantly affect time to progression to CRPC (p > 0.05).
Discussion
Testosterone elevated above the castrate range during ADT is referred to as testosterone escape.4 There are two types of testosterone elevations during ADT: acute-on-chronic responses and breakthrough responses. Acute-on-chronic responses are testosterone elevations that occur shortly after LHRH agonist administration, excluding the initial LHRH administration and testosterone flare. These responses arise because of direct action of LHRH agonist on the LHRH receptor and have an uncertain clinical significance.4
Breakthrough elevations occur when testosterone production recommences in the setting of continued LHRH agonist administration due to suboptimal drug effect.13 Testosterone escape is the subject of the current study. In this study, we found that 9-month and 1-year average testosterone levels below 32 ng/dL predicted a longer time to CRPC progression. These findings suggest that a lower threshold for castration might be used to predict time to CRPC in men on ADT. Whether manipulating androgens to target lower testosterone levels might yield a survival benefit remains to be adequately evaluated.
The apparent significance of a testosterone threshold of 32 ng/dL in this study reinforces findings from Morote and colleagues.12 These authors studies 73 patients with non-metastatic prostate cancer receiving 3-month depots of LHRH agonist. Serum testosterone was determined 3 times in 6 months and patients were stratified to breakthrough groups of 50 ng/dL, 32 ng/dL and 20 ng/dL. After a median follow-up of 51 months, 41 patients had progressed to CRPC. The authors found testosterone breakthroughs above 50 ng/dL and 32 ng/dL predicted progression to CRPC. These data are also interesting because patients with a breakthrough testosterone above 50 ng/dL had a significantly improved freedom from CRPC if treated with bicalutamide.12 As in our study, breakthroughs above 20 ng/dL were not significantly associated with time to CRPC.
Perachino and colleagues have also published an important series on this topic.11 Their retrospective series included 129 previously untreated bone-only metastatic prostate cancer patients who received 3-month depots of goserelin. PSA and testosterone were determined every 3 months. After a mean follow-up of 47.5 months, 58 (45%) patients were alive. Median testosterone at 6 months was 29 ng/dL (range: 17.5–50.5). Using multivariate Cox regression analysis, cancer-specific mortality was predicted by Gleason score, 6-month PSA levels and 6-month serum testosterone levels (HR 1.32, p < 0.05).
Limitations in this study are similar to those of previous studies.11,12 Firstly, limitations arise from the accuracy of the chemiluminescent testosterone assay due to potential cross-reactivity with other steroid hormones.17 Nonetheless, chemiluminescent assays have previously been found to have a low enough inter-assay variation coefficient to allow for the measurement of individual variations of serum testosterone in the castrate range (<50 ng/dL).18 An alternative assay would involve liquid chromatography and tandem mass spectrometry, which would provide excellent sensitivity, accuracy and specificity.17 Some authors have employed these techniques,19 but due to their limited accessibility they have not yet been employed in other similar studies.11,12
This study is also limited by its small sample size. While this study was designed and the data collected prospectively, the inclusion of a testosterone threshold of 32 ng/dL was made post-hoc. Results from Morote and colleagues12 were not available at the time of study commencement, and hence this study suffers from inherent limitations of post-hoc designs. The lack of standardization of androgen deprivation in this study (i.e., the variable use of LHRH agonists and antagonists) represents another limitiation. In spite of these limitations, the hypothesis that testosterone levels are important in predicting clinical outcomes is supported by other authors.11,12
The necessity of routinely measuring testosterone during ADT is controversial. In a 2005 survey of 400 urologists, 29% stated they did not know the testosterone levels of their patients and 49% stated that they knew the levels of only a few patients.20 Respondents who felt that castrate levels should be 50 ng/dL and 20 ng/dL were 31% and 64%, respectively.20 Similarly, in a recent survey of 113 Canadian practitioners, only 24% indicated that they measured testosterone routinely and half of respondents (53%) believed 50 ng/dL was an adequate castrate testosterone level.21
Indirect high-quality evidence also supports the role of additional modulation and monitoring of testosterone in prostate cancer. Firstly, prostate cancer can be prevented by decreasing dihydrotestosterone in the prostate.22,23 The impact of serum testosterone levels on prostate cancer is further supported by the survival benefit afforded by newer CRPC agents. Abiraterone inhibits the cytochrome P450 c17 enzyme, a critical enzyme in testosterone synthesis.24 This inhibition results in testosterone concentrations of 1 to 2 ng/dL, much lower than achievable by other castration methods.24 A survival benefit has been demonstrated in the post-chemotherapy setting.24 Additionally, an interim analysis of a trial involving the use of abiraterone in the pre-chemotherapy setting demonstrated improvements in progression-free survival, overall survival and quality of life outcomes.25 Preliminary phase-II studies also suggest that abiraterone used in the neoadjuvant setting may induce total or near pathological complete responses in patients with high-risk prostate cancer.26 MDV3100 is another newer CRPC agent that inhibits the action of the androgen receptor27 that has also demonstrated a survival benefit in post-chemotherapy CRPC.28
Conclusion
Both direct and indirect evidence make it clear that there is a need for larger prospective studies involving the measurement of testosterone levels during ADT. Ideally, these studies should incorporate both LHRH agonist/antagonist agents, as well as newer modulators of the androgen axis.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;167(2 Pt 2):948–51. doi: 10.1016/S0022-5347(02)80307-X. discussion 952. [DOI] [PubMed] [Google Scholar]
- 2.Murphy WM, Soloway MS, Barrows GH. Pathologic changes associated with androgen deprivation therapy for prostate cancer. Cancer. 1991;68:821–8. doi: 10.1002/1097-0142(19910815)68:4<821::AID-CNCR2820680426>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Civantos F, Marcial MA, Banks ER, et al. Pathology of androgen deprivation therapy in prostate carcinoma. A comparative study of 173 patients. Cancer. 1995;75:1634–41. doi: 10.1002/1097-0142(19950401)75:7<1634::AID-CNCR2820750713>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Djavan B, Eastham J, Gomella L, et al. Testosterone in prostate cancer: the Bethesda consensus. BJU Int. 2012;110:344–52. doi: 10.1111/j.1464-410X.2011.10719.x. . Epub 2011 Nov 30. [DOI] [PubMed] [Google Scholar]
- 5.Tombal B. Appropriate Castration with Luteinising Hormone Releasing Hormone (LHRH) Agonists: What is the Optimal Level of Testosterone? European Urology Supplements. 2005;4:14–9. doi: 10.1016/j.eursup.2005.04.004. [DOI] [Google Scholar]
- 6.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–4. doi: 10.1016/S0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 7.Schally AV, Arimura A, Baba Y, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43:393–9. doi: 10.1016/0006-291X(71)90766-2. [DOI] [PubMed] [Google Scholar]
- 8.Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and in otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem. 1987;33:1372–5. [PubMed] [Google Scholar]
- 9.Schulman CC, Irani J, Morote J, et al. Testosterone measurement in patients with prostate cancer. Eur Urol. 2010;58:65–74. doi: 10.1016/j.eururo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler MJ, D’Souza A, Matadeen J, et al. Ciba Corning ACS:180 testosterone assay evaluated. Clin Chem. 1996;42:1445–9. [PubMed] [Google Scholar]
- 11.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648–51. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 12.Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178(4 Pt 1):1290–5. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 13.Tombal B, Berges R. Optimal Control of Testosterone: A Clinical Case-Based Approach of Modern Androgen-Deprivation Therapy. European Urology Supplements. 2008;7:15–21. doi: 10.1016/j.eursup.2007.11.001. [DOI] [Google Scholar]
- 14.National Comprehensive Cancer Network (U.S.) The complete library of NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network; Rockledge, PA: 2003. [DOI] [PubMed] [Google Scholar]
- 15.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–83. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–95. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 17.van der Sluis TM, Vis AN, van Moorselaar RJ, et al. Intraprostatic testosterone and dihydrotestosterone. Part I: concentrations and methods of determination in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109:176–82. doi: 10.1111/j.1464-410X.2011.10651.x. [DOI] [PubMed] [Google Scholar]
- 18.Morote J, Planas J, Salvador C, et al. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2009;103:332–5. doi: 10.1111/j.1464-410X.2008.08062.x. discussion 335. [DOI] [PubMed] [Google Scholar]
- 19.van der Sluis TM, Bui HN, Meuleman EJ, et al. Lower testosterone levels with luteinizing hormone-releasing hormone agonist therapy than with surgical castration: new insights attained by mass spectrometry. J Urol. 2012;187:1601–6. doi: 10.1016/j.juro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 20.Zlotta A, Debruyne FMJ. Expert Opinion on Optimal Testosterone Control in Prostate Cancer. European Urology Supplements. 2005;4:37–41. doi: 10.1016/j.eursup.2005.08.005. [DOI] [Google Scholar]
- 21.Nora SD, Shayegan B. Inconsistent Testosterone Monitoring and Antiandrogen Use in Prostate Cancer Patients Receiving Androgen Deprivation Therapy [abstr UP-050] Can Urol Assoc J. 2012;6:S83. [Google Scholar]
- 22.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 23.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan CJ, Smith MR, De Bono JS, et al. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2012;30 [suppl; abstr LBA4518]. [Google Scholar]
- 26.Taplin M-E, Montgomery RB, Logothetis C, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. J Clin Oncol. 2012;30 doi: 10.1200/JCO.2013.53.4578. [suppl; abstr 4521]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–8. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 28.Scher HI, Fizazi K, Saad F, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. J Clin Oncol. 2012;30 [suppl 5; abstr LBA1]. [Google Scholar]