Abstract
Rab GTPases regulate vesicular traffic in eukaryotic cells by cycling between the active GTP-bound and inactive GDP-bound states. Their functions are modulated by the diverse selection of effector proteins that bind to specific Rabs in their activated state. We previously described the expression of Rab13 in bone cells. To search for novel Rab13 interaction partners, we screened a newborn rat bone marrow cDNA library for Rab13 effectors with a bacterial two-hybrid system. We found that Rab13 binds to the C-terminus of Endospanin-2, a small transmembrane protein. In addition to Rab13 also Rab8 bound to Endospanin-2, while no binding of Rab7, Rab10, Rab11 or Rab32 was observed. Rab13 and Rab8 also interacted with Endospanin-1, a close homolog of Endospanin-2. Rab13 and Endospanin-2 colocalised in perinuclear vesicular structures in Cos1 cells suggesting direct binding also in vivo. Endospanin-2 is implicated in the regulation of the cell surface growth hormone receptor (GHR), but the inhibition of Rab13 expression did not affect GHR cell surface expression. This suggests that the Rab13–Endospanin-2 interaction may have functions other than GHR regulation. In conclusion, we have identified a novel interaction for Rab13 and Rab8 with Endospanin-2 and Endospanin-1. The role of this interaction in cell physiology, however, remains to be elucidated.
Keywords: Vesicle trafficking, Rab13, Rab effector, Protein interaction, Endospanin, Osteoclast
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; GHR, growth hormone receptor; GST, glutathione-S-transferase; HA, human influenza hemagglutinin; MBP, maltose binding protein; OB-R, leptin receptor; VPS55, vacuolar protein sorting 55.
Highlights
▸ Rab13 and Rab8 both interact with Endospanin-2 and Endospanin-1. ▸ Rab13 and Rab8 binding to endospanins is specific; Rabs 7, 10, 11 and 32 do not bind. ▸ Rab13 binding to Endospanin-2 is nucleotide-dependent. ▸ Rab13 and Endospanin-2 colocalise in perinuclear vesicles and at the cell periphery.
1. Introduction
Vesicular trafficking is precisely monitored in eukaryotic cells to maintain specific features of intracellular organelles and membrane domains. Rab proteins constitute the largest subfamily of Ras small GTPases and more than 60 Rabs in humans and 11 in yeast [1] have been identified. They are important regulators of all steps of vesicular trafficking, including vesicle formation, movement along cytoskeletal tracks, tethering and vesicle fusion to the target membrane [2].
Rab proteins cycle between their membrane-associated activated GTP-bound and cytosolic inactivated GDP-bound conformations. They bind to several types of proteins that regulate their activity or membrane association and further increase the specificity of their function. Rab effectors are functionally defined as molecules that selectively bind to specific Rabs in their GTP-bound state and modulate their downstream effects [2]. Diverse functions of Rabs in different cell types or in distinct vesicular pathways within cells are most likely defined by the unique set of effectors present.
Rab13 is a small GTPase with an array of functions in different cell types, from regulation of tight junctions to neuronal plasticity, cell migration and glucose transporter trafficking [3–6]. We have previously described the expression of Rab13 in bone cells including osteoclasts. In bone-resorbing osteoclasts Rab13 is located in small vesicular structures between the trans Golgi network and the non-bone facing plasma membrane. It is not involved in bone degradation process, nor associates with early or recycling endosomes. The vesicle type and the cargo of Rab13-positive vesicles in osteoclasts remain still to be identified [7]. In this study, we screened for novel Rab13 effectors in rat bone marrow and describe the interaction of Rab13 with Endospanin-2, a small transmembrane protein.
2. Materials and methods
2.1. Antibodies
Goat polyclonal GST and mouse monoclonal β-actin antibodies were purchased from Sigma–Aldrich. The BD living colours Full-length A.v. rabbit polyclonal antibody used to detect GFP-Rab fusion proteins was from Clontech Takara Bio. The mouse monoclonal Ha.11 antibody (clone 16B12) was obtained from Covance. Polyclonal rabbit Rab13 antibody was purchased from Atlas antibodies, and the mouse anti-rat β3-integrin monoclonal antibody was a generous gift from Dr. M.A. Horton, University College London, UK. Mouse monoclonal antibodies for the growth hormone receptors; mAb263 and Mab5 were purchased from Abcam and Santa Cruz, respectively. HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Dako and the HRP-conjugated anti-goat antibody was purchased from Zymed Laboratories. Alexa Fluor 488- or 546-conjugated secondary antibodies were purchased from Molecular Probes.
2.2. Cell lines and transfections
Easily transfectable Cos1 cells were used in immunoprecipitation and colocalisation studies, and HeLa cells were used in siRNA silencing studies. LNCaP cells (an androgen sensitive prostate adenocarcinoma cell line) were used in growth hormone receptor studies [8]. Cos1 and HeLa cells were cultured in DMEM (Gibco) supplemented with 10% inactivated foetal calf serum (iFCS, Gibco), Glutamax (Gibco) and penicillin/streptomycin (Gibco) at 37 °C under a 5% CO2 atmosphere. LNCaP cells were cultured in RPMI (Lonza) supplemented with 15% iFCS, Glutamax, Hepes (Lonza), sodium pyruvate (Lonza) and penicillin/streptomycin. Cos1 cells were transfected using Fugene 6 (Roche Diagnostics), HeLa cells were transfected using Lipofectamine 2000 (Invitrogen) and LNCaP cells were transfected using Amaxa Nucleofector (Lonza) with Amaxa Cell Line Nucleofector Kit R.
2.3. Plasmid constructs and siRNA molecules
Rat Rab13 and Rab7 reading frames were cloned into a pBT vector to generate the bait plasmids for the bacterial two-hybrid screen. The construction and cloning of the rat trabecular bone marrow cDNA library into a pTRG plasmid is described elsewhere [9]. Reading frames of rat Rab7, Rab13, Rab11 and Rab32 were cloned into a pGEX-4T-1 plasmid (Amersham Biosciences) to produce glutathione-S-transferase (GST)-Rab fusion proteins. The last 20 C-terminal amino acids of rat Endospanin-2 or Endospanin-1 were cloned into pMAL-c2e plasmid (New England Biolabs) to produce Maltose Binding Protein (MBP)-fusion proteins.
Human Rab13 and Rab32 reading frames were cloned into a pEGFP-Actin plasmid (Clontech Laboratories, Inc.) to produce GFP-tagged Rab eukaryotic overexpression vectors. A eukaryotic expression vector for full-length rat Endospanin-2 was generated by cloning the Endospanin-2 reading frame into a pcDNA3.1(Neo)(+)-plasmid (Invitrogen) with a human influenza hemagglutinin (HA)-tag (Tyr-Pro-Tyr-Asp-Val-Pro-Asp-Tyr-Ala) inserted into its first extracellular loop. Rab13 mutants defective in GTP hydrolysis (Rab13Q67L) or in GDP-to-GTP exchange (Rab13T22N) were generated with QuickChange Site-Directed Mutagenesis Kit (Stratagene). GFP-Rab8 and GFP-Rab10 eukaryotic expression vectors were from Yi Sun (Program in Cell Biology, The Hospital for Sick Children, Toronto, ON, Canada). The human Rab13 siRNA molecule (target sequence: cagggcaaacataaatgtaaa) and non-related negative control siRNA molecule were purchased from Qiagen.
2.4. Bacterial two-hybrid screen
pBT-Rab13Q67L was used as bait to screen the bone marrow cDNA library [9] in the Bacteriomatch II Two-Hybrid System with Electrocompetent Cells (Stratagene). Positive interactions were demonstrated by bacterial growth in the selective medium lacking histidine and in the presence of 5 mM 3-amino-1,2,4-triazole (Sigma). Positive interactions were verified in a secondary screen and validated by individual retransformation of purified target plasmids with the bait plasmids.
2.5. In vitro pull-down experiments
MBP- or GST-fusion proteins were produced by bacterial expression and cellular pellets were collected. Cells were suspended in binding buffer (50 mM Tris, 300 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 10 mM β-mercaptoethanol, 0.1% Triton X-100 and protease inhibitors (Roche), pH 7.2) and lysed by sonication. Equimolar amounts of MBP-bait proteins (∼10 μg) were incubated with GST-target proteins (∼2 μg) for 2 h at 4 °C together with amylose resin beads (New England BioLabs). The beads were washed, and bound proteins were eluted in 10 mM maltose and processed for western blotting.
MBP-fusion proteins were bound to amylose resin beads and incubated with HeLa or Cos1 cell lysates in binding buffer for 2 h at 4 °C. The beads were washed, and bound proteins were eluted and processed for western blot analysis.
2.6. Immunoprecipitation
Cos1 cells co-transfected with pCDNA-Endospanin-2HA and pEGFP constructs were lysed in binding buffer (50 mM Tris, 150 mM NaCl, phenylmethylsulfonyl fluoride, 0.5% Triton X-100 and protease inhibitors). Equal amounts of cell lysate were incubated with GFP antibody and protein A magnetic beads (Invitrogen) for 2 h at 4 °C. After being washed, the bound proteins were eluted in Laemmli buffer and subjected to immunoblotting.
LNCaP protein lysate was incubated with or without the mAb263 anti-growth hormone receptor (GHR) antibody together with protein G magnetic beads (Invitrogen) in binding buffer for 2 h at 4 °C. After a washing step, the bound proteins were eluted in Laemmli buffer and subjected to western blotting with an Mab5 anti-GHR antibody.
2.7. Immunofluorescence
Forty-eight hours post-transfection, Cos1 cells were rinsed with PBS and fixed in 3% paraformaldehyde. Free aldehyde groups were quenched in 50 mM NH4Cl and cells were permeabilised in 0.1% Triton-PBS on ice. Nonspecific binding was blocked with 2% BSA before incubation with an Ha.11 antibody. After washing steps, primary antibody binding was visualised using Alexa Fluor 488- or 546-labelled secondary antibodies. The cells were observed with a Leica TCS-SP confocal laser scanning microscope equipped with an Argon–Krypton laser (Leica Microsystems). Confocal images were acquired by sequential scanning.
2.8. Flow cytometry
Twenty-four hours after siRNA transfection, LNCaP cells were serum-starved for 20 h and detached using non-enzymatic Cell Dissociation Solution (Sigma–Aldrich). Cells were washed in ice-cold PBS containing 0.5% BSA and 2% normal goat serum and incubated with an anti-GHR antibody (mAb263) for 90 min on ice in the same buffer. The cells were washed three times, and treated with Alexa Fluor 488-conjugated anti-mouse antibodies. After being washed, 105 cells were analysed with a FACScan flow cytometer (Becton-Dickinson) and Cell Quest data acquisition and analysis software. Background fluorescence was excluded from the analysis by gating.
2.9. Osteoclast isolation, RNA purification and PCR
All animal experiments were carried out in accordance with EU Directive 2010/63/EU for animal experiments. Osteoclasts were isolated from the bone marrow of newborn rats with anti-integrin β3-coated magnetic beads as previously described [10]. RNA was purified using a Total RNA Isolation Kit (Qiagen), and mRNAs were reverse transcribed with Superscript III Reverse Transcriptase (Invitrogen). PCR reactions were incubated in a thermal cycler (Eppendorf) with Advantage 2 PCR Enzyme (Clontech Laborarories), the products were separated by 1% agarose electrophoresis, and the bands were visualised using ethidium bromide staining.
2.10. PCR primers
Used PCR primers were 5′-aaccaatactggcccctcttcgttc-3′ and 5′-agcgcttcaccattgctgccag-3′ for Endospanin-2, 5′-atgcaagcttgacatctt-3′ and 5′-tccgagccaagagaacat-3′ for cathepsin K and 5′-accacagtccatgccatcac-3′ and 5′-tccaccaccctgttgctgta-3′ for glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
2.11. Software
Endospanin-2 transmembrane domains were predicted with TMHMM software [11] and the schematic drawn with DOG 2.0 software [12]. Determination of the Pearson's correlation coefficient and densitometric analysis of the co-immunoprecipitation data were done with ImageJ software.
3. Results
3.1. Endospanin-2 identified as a Rab13-binding protein
To find novel Rab13 interaction partners, we screened a newborn rat bone marrow cDNA library [9] with Rab13Q67L, a dominant active mutant of Rab13, as bait in a bacterial two-hybrid system. The initial screen consisted of approximately 2 × 106 transformants, of which 1889 clones demonstrated positive growth. After validation of the interactions, we found a possible candidate in several copies. One of these clones, pTRG-3D3, was studied in more detail. When target plasmid pTRG-3D3 was co-transformed with Rab13WT or Rab13Q67L-mutant bait plasmids, a positive interaction was observed, whereas co-transfectants with empty bait plasmid or a bait plasmid encoding for Rab7 did not grow (Fig. 1A). This result indicates that the interaction of Rab13 with this clone is specific. Analysis of this clone revealed that it contained a 353-base pair insert that coded for 20 amino acids (TILGFFLVFGSNDDFSWQQW) that are identical to the last C-terminal 20 amino acids of Endospanin-2. Endospanin-2 is a small protein that contains four hydrophobic transmembrane domains, short cytosolic N- and C-termini and two extracellular loops [13,14]. Of the 20 amino acids found in pTRG-3D3, the first nine belong to the last transmembrane domain, and the last 11 form the cytosolic C-tail (Fig. 1B). Endospanin-2 belongs to the VPS55 protein family that consists of two members in mammals, Endospanin-2 and Endospanin-1 which share 67% homology in humans [13]. Endospanin-2 and Endospanin-1 were previously named as Leptin receptor overlapping transcript like-1 (LeprotL1) and Obesity gene-related protein (Ob-rgrp or Leptin receptor overlapping transcript, Leprot), respectively [14].
Fig. 1.
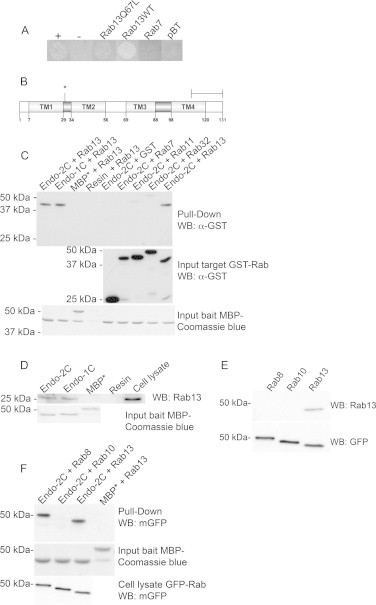
Rab13 binding to the Endospanin-2 C-terminal tail is specific. (A) Bacterial transformants carrying the target vector pTRG-3D3 and the bait pBT vectors encoding Rab13Q67L, Rab13WT, Rab7 or empty pBT vector were cultured in selective minimal medium. Positive and negative controls were from Bacteriomatch II Two-Hybrid System. Positive interaction was indicated by bacterial growth in the selective medium. (B) A schematic of Endospanin-2. TM indicates transmembrane domain, dark grey and white indicate extracellular and intracellular domains, respectively. Scale bar indicates the C-terminal tail identified as Rab13 binding in bacterial two-hybrid system. Asterisk marks the insertion site of the HA tag in Endospanin-2HA overexpression construct. (C) Bacterially expressed GST-Rab13, GST-Rab7, GST-Rab11 and GST-Rab32 proteins were precipitated with C-terminal tails of Endospanin-2 (Endo-2C) or Endospanin-1 (Endo-1C) fused to MBP and subjected to immunoblotting. Negative controls were GST-Rab13 with MBP* or empty amylose resin beads and MBP-Endo-2C with GST. (D) Bacterially expressed MBP-Endo-2C and MBP-Endo-1C were bound to amylose resin beads and incubated with HeLa cell lysates. Retained proteins were analysed by western blotting. (E) The specificity of the polyclonal Rab13 antibody was validated with Cos1 cell lysates transfected with GFP-Rab8, 10 and 13 eukaryotic expression vectors. (F) MBP-Endo-2C was bound to amylose resin beads and incubated with GFP-Rab8, 10 or 13 transfected Cos1 cell lysates. Retained proteins were analysed by western blotting. Equal protein inputs were confirmed by immunoblotting and/or Coomassie blue staining. Calculated molecular mass values are: MBP-Endospanin-2 and Endospanin-1, 45 kDa; MBP* (MBP4*-β-gal α fragment from pMal-c2E plasmid with no insert), 51 kDa; GST, 26 kDa; GST-Rab7, 50 kDA; GST-Rab11, 51 kDa; GST-Rab13, 49 kDa; GST-Rab32, 52 kDa; endogenous Rab13, 23 kDa; GFP-Rab8, 50 kDa; GFP-Rab10, 49 kDa and GFP-Rab13, 49 kDa.
3.2. In vitro binding of Rab13 to Endospanin-2
The bacterial two-hybrid interaction between Rab13 and Endospanin-2 was confirmed in a pull-down assay with bacterially expressed recombinant proteins. As bait, we used the last 20 amino acids of Endospanin-2 (Endo-2C in Fig. 1) or a similar construct with the last 20 amino acids of Endospanin-1 (Endo-1C in Fig. 1). GST-fused Rab13 bound to both MBP-Endo-2C and MBP-Endo-1C, whereas no binding was observed to the negative controls, MBP or empty amylose resin beads. GST alone did not bind MBP-Endo-2C, nor did GST-Rab7, 11 or 32 (Fig. 1C). Bacterially expressed MBP-Endo-2C and MBP-Endo-1C also precipitated endogenous Rab13 from HeLa cell lysates (Fig. 1D). This signal is Rab13-specific since the used Rab13 antibody did not recognise GFP tagged Rab8 or Rab10 from transfected Cos1 cell lysates (Fig. 1E). MBP-Endo-2C was able to precipitate GFP-Rab8 and GFP-Rab13, but not GFP-Rab10, from transfected Cos1 (Fig. 1F) or HeLa (data not shown) cell lysates. GFP-Rab8 also bound to the C-terminal tail of Endospanin-1, while GFP-Rab10 did not (data not shown). These results indicate that Rab13 and its homolog, Rab8, bind to the C-terminal tails of Endospanin-2 and Endospanin-1.
3.3. Interaction of Rab13 and Endospanin-2 in a cellular environment
We were not able to study endogenous Endospanin-2 due to lack of functional antibodies. Instead, we generated a eukaryotic overexpression vector for full-length Endospanin-2 with a HA-tag inserted into its first extracellular loop (Endospanin-2HA) (Fig. 1B). We transiently co-transfected Cos1 cells with Endospanin-2HA together with different combinations of GFP-tagged Rabs, and then immunoprecipitated protein complexes with an anti-GFP antibody. Congruent with the bacterially expressed proteins, we observed binding of Endospanin-2HA to the GFP-Rab13WT but no binding to the GFP-Rab32 (Fig. 2A).
Fig. 2.
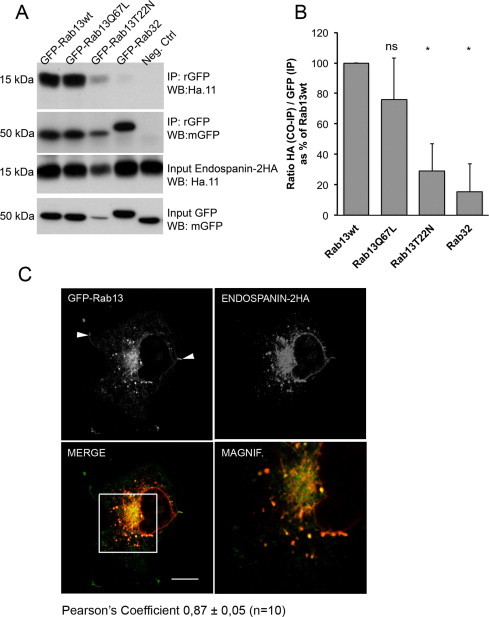
Endospanin-2 associates with Rab13WT and Rab13Q67L in cellular systems. (A) Cos1 cells were transiently transfected with Endospanin-2HA and GFP-Rab13WT, GFP-Rab13Q67L, GFP-Rab13T22N or GFP-Rab32 constructs, lysed after 48 h, immunoprecipitated with an anti-GFP antibody and subjected to western blot analysis with anti-HA and anti-GFP antibodies. The negative control was Endospanin-2HA- and GFP-Rab13WT-transfected cell lysates without the anti-GFP antibody. (B) Densitometric analysis of three independent co-immunoprecipitation experiments (mean + standard deviation). Student's t-test shows no statistical significance for Rab13WT and Rab13Q67L binding to Endospanin-2HA. (C) Cos1 cells were co-transfected with GFP-Rab13WT and Endospanin-2HA and labelled with Ha.11 antibody. Rab13 (green) and Endospanin-2HA (red) were colocalised (yellow) in vesicles at the perinuclear area and at the cell periphery. Rab13 showed clear plasma membrane staining (white arrowheads), whereas few Endospanin-2HA-positive vesicles were detected at the cell periphery. Square box displays the magnified field. Scale bars, 10 μm. Densitometric analysis and the determination of the Pearson's correlation coefficient (n = 10 cells) were done with ImageJ software. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Effectors typically bind to Rabs in a nucleotide-dependent manner [2]. The binding of Endospanin-2HA to the active form of Rab13 (GFP-Rab13Q67L) was similar to that of wild type Rab13, whereas binding to the inactive form of Rab13 (GFP-Rab13T22N) was weaker (Fig. 2A and B). It should be noted, however, that the protein expression levels in these cells are not uniform. GFP-tagged Rab13 mutant proteins are difficult to express also in other cellular systems [3]. These results show that the full-length Endospanin-2 binds to Rab13 wild type and active (GTP-bound) forms.
Cos1 cells were co-transfected with Endospanin-2HA and GFP-Rab13WT constructs and visualised with Ha.11 immunofluorescence staining. Endospanin-2HA demonstrated a punctate vesicular staining pattern across the cell with strong clusters of labelling at perinuclear areas. Some vesicular structures were labelled at or near the plasma membrane. GFP-Rab13WT localised to perinuclear vesicular structures, but unlike for Endospanin-2HA, a clear plasma membrane staining was observed (white arrowheads in Fig. 2B). These localisations are congruent with the previous reports of endogenous Rab13 and Endospanin-2 [14,15]. In addition to punctate, vesicular-like staining patterns of these proteins, we observed staining of the nuclear envelope for Endospanin-2HA and ER-type reticular labelling for both proteins.
GFP-Rab13WT and Endospanin-2HA colocalised in vesicular structures, although the association was not complete (Fig. 2B). Strong colocalisation was detected at the perinuclear structures and vesicles arising from this area. Colocalisation of these two proteins was weaker at the cell periphery. These results indicate that in addition to the direct binding of Rab13 and Endospanin-2 in vitro, they colocalise in the same cellular structures, suggesting direct binding in vivo.
Next, we tested if inhibition of Rab13 expression affects Endospanin-2 localisation. We co-transfected HeLa cells with the expression vector for Endospanin-2HA together with either control or Rab13 siRNA molecules. Rab13 siRNA treatment reduced the expression of endogenous Rab13 protein by 80% compared to the control siRNA-treated cells (Fig. 3A; quantitation with ImageJ not shown). Control siRNA-transfected cells displayed a distribution of Endospanin-2HA similar to that observed in Cos1 cells. Endospanin-2HA was located on vesicular structures throughout the cells, with the strongest labelling found at the perinuclear structures. Some vesicles were also observed at the cell periphery close to the plasma membrane (Fig. 3B). Rab13 siRNA-transfected cells displayed a similar distribution of Endospanin-2HA, and we observed no evident difference compared to the control siRNA-transfected cells (Fig. 3B). Morphological transport assay where transfected cells are cultured in 20 °C to block the transfer of proteins from the TGN after which the block is released by incubation at 37 °C for different time periods could not differentiate Rab13 siRNA-transfected cells from control siRNA-transfected cells (not shown). We also detected very little – if any – of endocytosed Endospanin-2HA in antibody uptake experiments (not shown).
Fig. 3.
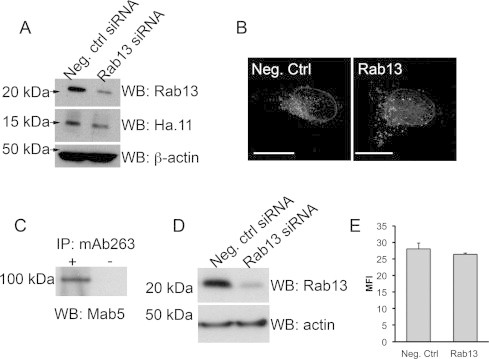
Effect of rab13 downregulation on Endospanin-2HA localisation and cell surface expression of GHR. (A) Rab13 siRNA molecules downregulated Rab13 protein expression by 80% in HeLa cells (quantitation with ImageJ not shown). (B) The distribution of Endospanin-2HA in control siRNA- and Rab13 siRNA-transfected HeLa cells. Shown are extended focus images reconstructed from the original confocal sections by adding 20 sections taken at 0.117 μm intervals together. Scale bar, 10 μm. (C) GHR expression in LNCaP cells, as demonstrated by immunoprecipitation with an anti-GHR (mAb263) antibody. (D) Rab13 siRNA treatment reduced the expression of Rab13 protein by 80% in LNCap cells (quantitation with ImageJ not shown). (E) siRNA-transfected LNCaP cells were incubated with mAb263 (anti-GHR) and an Alexa Fuor 488-conjugated secondary antibody and analysed by flow cytometry. Data are represented as average mean fluorescent intensities with standard deviations (MFI + SD) from four independent experiments. Student's t-test shows no statistical significance.
Endospanin-2 and Endospanin-1 were described as negative regulators of cell surface expression of GHR and leptin receptor (OB-R) [14,16,17]. To test whether Rab13 is involved in the regulation of GHR expression, we studied the cell surface expression of GHR in LNCaP cells, which have functional endogenous GHR and growth hormone signalling pathways [8]. GHR expression in LNCaP cells was first confirmed by immunoprecipitation of the receptor (Fig. 3C). Rab13 siRNA transfection by electroporation reduced Rab13 expression by 80% in these cells (Fig. 3D; quantitation with ImageJ not shown). Flow cytometry analysis with the anti-GHR (mAb263) antibody showed that there was no difference in cell surface GHR levels between Rab13 siRNA-treated and control siRNA-treated cells, suggesting that the Rab13–Endospanin-2 interaction may have other functions (Fig. 3E). Similar results were obtained from growth hormone treated LNCaP cells (not shown). The observed fluorescence intensities were similar to the intensities reported previously in HEK293 cells overexpressing GHR [18].
3.4. Endospanin-2 is expressed in osteoclasts
Because we have previously described the expression of Rab13 in osteoclasts of rat and human origin, we next studied the expression of Endospanin-2 in rat osteoclasts. Due to the lack of functional antibodies against the endogenous Endospanin-2 we analysed its expression in primary rat osteoclasts at mRNA level using gene specific primer pairs. Rat osteoclast enrichment by affinity purification was successful, as confirmed by PCR amplification of cathepsin K mRNA. The expression of Endospanin-2 mRNA was detected in osteoclasts, as well as in non-osteoclasts and bone marrow cell populations (Fig. 4).
Fig. 4.
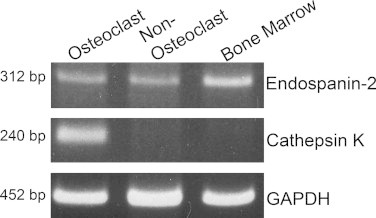
Endospanin-2 is expressed in rat osteoclasts. Total RNA was isolated from rat osteoclasts enriched by magnetic separation with a β3-integrin antibody from newborn rat bone marrow (Osteoclasts) and the remaining cell population (Non-Osteoclasts). After reverse transcription, Endospanin-2, Cathepsin K and GAPDH were amplified with gene specific primer pairs by PCR.
4. Discussion
Rab13 is a small GTPase regulating the formation and maintenance of tight junctions in epithelial cells [19–21], and it has recently been shown to have more versatile functions in different cell types: it regulates the biosynthetic trafficking of cell surface proteins between the trans-Golgi network (TGN) and the recycling endosomes in polarised MDCK cells [22], and in neurons, Rab13 regulates neurite outgrowth and regeneration after injury [4,23]. Interestingly, in L6 muscle cells Rab13 is activated by insulin and thereby increases the translocation of glucose transporter-4 to the cell surface [5].
We demonstrated the expression of Rab13 in osteoclasts and other bone marrow cells, but its function in these cells remains unknown [7]. In our screen for novel Rab13-interacting proteins, we identified a new highly conserved Rab13-interacting protein family with two members in mammals, Endospanin-2 and Endospanin-1. These proteins are small, 131-amino acid proteins that contain four transmembrane domains and intracellular N- and C-termini. The yeast genome has only one homolog of this protein family, VPS55, which is suggested to cycle between late endosomes and the TGN [24]. In mammalian cells, Endospanin-2 and Endospanin-1 are localised to distinct cellular domains; the former is perinuclear and is associated with the TGN, while the latter is located in late endosomes at the cell periphery [14]. The distinct distributions of Endospanin-2 and Endospanin-1 imply that these proteins may have divergent functions in higher organisms. The intracellular location of the Rab13–Endospanin-2 interaction suggests that this interaction has a role in the biosynthetic route, as both proteins are enriched close to the TGN area. Rab13 was recently described to partially localise to Rab7-labelled late endosomes in nonpolarised cells [25]. Therefore, Rab13 may also be involved in the endocytic route.
Endospanin-2 and Endospanin-1 are implicated as negative regulators of cell surface expression of OB-R and GHR by regulating the lysosomal degradation of endocytosed receptors [14,16,17]. However, in our experiments, knockdown of Rab13 had no apparent effect on endogenous cell surface GHR localisation in growth hormone treated (data not shown) or non-treated LNCaP cells, suggesting that Rab13–Endospanin-2 interaction may have additional functions that are unrelated to GHR trafficking. Alternatively, as only a small portion of the GHR and OB-R reach the cell surface, while the bulk is retained in intracellular structures such as the Golgi, ER and TGN [18,26–28], the novel interaction we found may be important for the intracellular distribution of these receptors.
Rab13 belongs to the same Rab subfamily as Rab8 [29] that also binds to Endospanin-2 and Endospanin-1. Rab13 and Rab8 are involved in similar processes, including the translocation of GLUT4 in muscle cells and the transport of junctional proteins to the tight junctions and adherens junctions [5,30]. In addition, Rab13 and Rab8 are reported to have several common binding partners, including MICAL-L1 and MICAL-L2/JRAB [25,30]. However, no compensatory function has been described, and Rab8 and Rab13 seem to function merely in a cascade [5]. It may however be, that in case of binding to Endospanin-2 such a compensatory redundancy exists, as we did not observe changes in the distribution of Endospanin-2HA when Rab13 was depleted. It is also possible that the achieved 80% reduction in protein levels is not enough to produce visible change in distribution of overexpressed proteins, i.e., the remaining 20% Rab13 is still enough to carry out function because of the high protein–protein affinity involved. Alternatively, Endospanin-2 may regulate the localisation of Rab13. Indeed, small four-transmembrane proteins with intracellular N- and C-termini, such as PRA proteins in mammals and Yip in yeast, recruit certain Rabs to specific membrane compartments [31–33]. We did not observe any clear effect of Endospanin-2HA overexpression on Rab13 localisation (not shown). Thus, it remains to be elucidated if Endospanin-2 indeed recruits Rab13 to vesicles in which it resides in. Rab13 regulates the redistribution of protein complexes involved in directional migration from vesicular structures to the leading edge in endothelial cells under VEGF gradient [6]. It is tempting to speculate that a similar induction, although the signal is still unknown, may be needed to reveal the relationships between Rab13 and Endospanin-2 intracellular distributions.
In conclusion, we found a novel interaction of Rab13 and Rab8 with Endospanin-2 and its homolog Endospanin-1. Endospanin-2 does not bind to Rab7, Rab10, Rab11 or Rab32 associated with late endosomes, Golgi, recycling compartments and melanin secretory vesicles, respectively [1]. Rab13 and Endospanin-2 colocalise in vesicles at the perinuclear area and, to a lesser extent, at the cell periphery. The role of this novel protein–protein interaction in cell physiology, in particular in osteoclasts, remains to be elucidated.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by Turku Doctoral Programme of Biomedical Sciences and grants from Academy of Finland and Sigrid Juselius Foundation.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Hutagalung A.H., Novick P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosshans B.L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marzesco A.M., Dunia I., Pandjaitan R., Recouvreur M., Dauzonne D., Benedetti E.L., Louvard D., Zahraoui A. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol. Biol. Cell. 2002;13:1819–1831. doi: 10.1091/mbc.02-02-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giovanni S., De Biase A., Yakovlev A., Finn T., Beers J., Hoffman E.P., Faden A.I. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J. Biol. Chem. 2005;280:2084–2091. doi: 10.1074/jbc.M411975200. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y., Bilan P.J., Liu Z., Klip A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc. Natl. Acad. Sci. USA. 2010;107:19909–19914. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Agrawal S., Vasanji A., Drazba J., Sarkaria S., Xie J., Welch CM., Liu M., Anand-Apte B., Horowitz A. Rab13-dependent trafficking of RhoA is required for directional migration and angiogenesis. J. Biol. Chem. 2011;286:23511–23520. doi: 10.1074/jbc.M111.245209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirvonen M.J., Mulari M.T.K., Büki K.G., Vihko P., Härkönen P.L., Väänänen H.K. Rab13 is upregulated during osteoclast differentiation and associates with small vesicles revealing polarized distribution in resorbing cells. J. Histochem. Cytochem. 2012;60:537–549. doi: 10.1369/0022155412448069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss-Messer E., Merom O., Adi A., Karry R., Bidosee M., Ber R., Kaploun A., Stein A., Barkey R.J. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol. Cell. Endocrinol. 2004;220:109–123. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y., Buki K.G., Ettala O., Vaaraniemi J.P., Vaananen H.K. Possible role of direct Rac1–Rab7 interaction in ruffled border formation of osteoclasts. J. Biol. Chem. 2005;280:32356–32361. doi: 10.1074/jbc.M414213200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H., Ettala O., Vaananen H.K. Intracellular membrane trafficking pathways in bone-resorbing osteoclasts revealed by cloning and subcellular localization studies of small GTP-binding rab proteins. Biochem. Biophys. Res. Commun. 2002;293:1060–1065. doi: 10.1016/S0006-291X(02)00326-1. [DOI] [PubMed] [Google Scholar]
- 11.Möller S., Croning M.D., Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics. 2001;17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 12.Ren J., Wen L., Gao X., Jin C., Xue Y., Yao X. DOG 1.0: illustrator of protein domain structures. Cell Res. 2009;19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y., Ying K., Xie Y., Zhou Z., Wang W., Tang R., Zhao W., Zhao S., Wu H., Gu S., Mao Y. Cloning and characterization of a novel human leptin receptor overlapping transcript-like 1 gene (LEPROTL1) Biochim. Biophys. Acta. 2001;1517:327–331. doi: 10.1016/s0167-4781(00)00266-9. [DOI] [PubMed] [Google Scholar]
- 14.Séron K., Couturier C., Belouzard S., Bacart J., Monté D., Corset L., Bocquet O., Dam J., Vauthier V., Lecœur C., Bailleul B., Hoflack B., Froguel P., Jockers R., Rouillé Y. Endospanins regulate a postinternalization step of the leptin receptor endocytic pathway. J. Biol. Chem. 2011;286:17968–17981. doi: 10.1074/jbc.M111.224857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahraoui A., Joberty G., Arpin M., Fontaine J.J., Hellio R., Tavitian A., Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J. Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couturier C., Sarkis C., Séron K., Belouzard S., Chen P., Lenain A., Corset L., Dam J., Vauthier V., Dubart A., Mallet J., Froguel P., Rouillé Y., Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2007;104:19476–19481. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touvier T., Conte-Auriol F., Briand O., Cudejko C., Paumelle R., Caron S., Baugé E., Rouillé Y., Salles J.-P., Staels B., Bailleul B. LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice. J. Clin. Invest. 2009;119:3830–3838. doi: 10.1172/JCI34997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milward A., Metherell L., Maamra M., Barahona M.J., Wilkinson I.R., Camacho-Hübner C., Savage M.O., Bidlingmaier M., Bidlingmaier C.M., Clark A.J.L., Ross R.J.M., Webb S.M. Growth hormone (GH) insensitivity syndrome due to a GH receptor truncated after Box1, resulting in isolated failure of STAT 5 signal transduction. J. Clin. Endocrinol. Metab. 2004;89:1259–1266. doi: 10.1210/jc.2003-031418. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y., Nishimura N., Morimoto S., Kitamura H., Manabe S., Kanayama HO., Kagawa S., Sasaki T. Distinct roles of Rab3B and Rab13 in the polarized transport of apical, basolateral, and tight junctional membrane proteins to the plasma membrane. Biochem. Biophys. Res. Commun. 2003;308:270–275. doi: 10.1016/s0006-291x(03)01358-5. [DOI] [PubMed] [Google Scholar]
- 20.Kohler K., Louvard D., Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J. Cell Biol. 2004;165:175–180. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto S., Nishimura N., Terai T., Manabe S., Yamamoto Y., Shinahara W., Miyake H., Tashiro S., Shimada M., Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 22.Nokes R.L., Fields I.C., Collins R.N., Fölsch H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J. Cell Biol. 2008;182:845–853. doi: 10.1083/jcb.200802176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakane A., Honda K., Sasaki T. Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2. Mol. Cell. Biol. 2010;30:1077–1087. doi: 10.1128/MCB.01067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belgareh-Touzé N., Avaro S., Rouillé Y., Hoflack B., Haguenauer-Tsapis R. Yeast Vps55p, a functional homolog of human obesity receptor gene-related protein, is involved in late endosome to vacuole trafficking. Mol. Biol. Cell. 2002;13:1694–1708. doi: 10.1091/mbc.01-12-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou-Zeid N., Pandjaitan R., Sengmanivong L., David V., Le Pavec G., Salamero J., Zahraoui A. MICAL-like1 mediates epidermal growth factor receptor endocytosis. Mol. Biol. Cell. 2011;22:3431–3441. doi: 10.1091/mbc.E11-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barr V.A., Lane K., Taylor S.I. Subcellular localization and internalization of the four human leptin receptor isoforms. J. Biol. Chem. 1999;274:21416–21424. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- 27.Belouzard S., Delcroix D., Rouillé Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J. Biol. Chem. 2004;279:28499–28508. doi: 10.1074/jbc.M400508200. [DOI] [PubMed] [Google Scholar]
- 28.Landsman T., Waxman D.J. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J. Biol. Chem. 2005;280:37471–37480. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- 29.Pereira-Leal J.B., Seabra M.C. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000;301:1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 30.Yamamura R., Nishimura N., Nakatsuji H., Arase S., Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol. Biol. Cell. 2008;19:971–983. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdul-Ghani M., Gougeon P.Y., Prosser D.C., Da-Silva L.F., Ngsee J.K. PRA isoforms are targeted to distinct membrane compartments. J. Biol. Chem. 2001;276:6225–6233. doi: 10.1074/jbc.M009073200. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa C., Taylor J., Vojtek A.B. Prenylated Rab acceptor protein is a receptor for prenylated small GTPases. J. Biol. Chem. 2001;276:28219–28225. doi: 10.1074/jbc.M101763200. [DOI] [PubMed] [Google Scholar]
- 33.Sivars U., Aivazian D., Pfeffer S. Yip3 catalyses the dissociation of endosomal Rab–GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]


