Fig. 2.
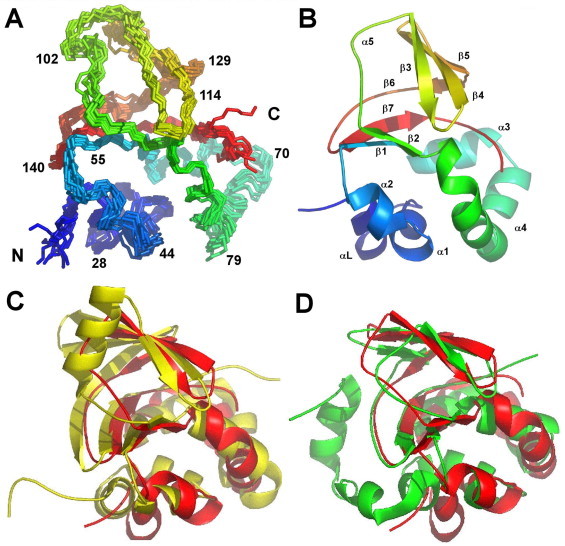
The solution structure of Fdp. (A) 10 overlaid structures, shown as a rainbow view, from blue at the N-terminus to red at the C-terminus. Only backbone atoms (Cα, C′, N) are shown. (B) Best structure as a cartoon, same colour scheme and orientation. The α-helix and β-sheet numbering is indicated. Labelling of helices and sheets follows that in [30]. This means that the first helix is αL rather than α1, α4 has a large bend in the middle, α5 is a helical turn rather than a full helix, and β6 is a short strand followed by a longer extended strand. (C) Best fit superposition to Drosophila FAS1-4 (Fdp red, FAS1 yellow). (D) Best fit superposition to M. tuberculosis MPB70 (Fdp red, MPB70 green). Superpositions and RMSD values in the text were based on the most highly conserved regions of secondary structure, corresponding to residues 45–50, 55–65, 82–94, 102–104, 114–122, 126–128 and 135–151 from Fdp. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
