Fig. 4.
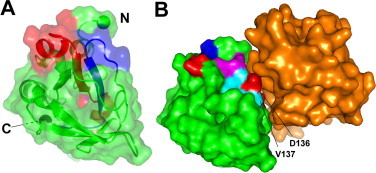
The binding surface on Fdp. (A) The surface of Fdp in partially transparent view. Conserved regions H1 and H2 are shown in red and blue respectively. They form a large patch at the top of the structure, run through the protein as two parallel β strands, and emerge on the opposite face. The N and C termini are indicated by spheres. (B) Suggested binding surface of Fdp; residues 50, 52 and 136–144, all other residues being green. Acidic residues are shown in red, basic in blue, hydrophobic in cyan, and hydrophilic in magenta. The key binding residues D136 and V137 are indicated. The orientation is the same as in (A). The orange surface is domain 3 of Drosophila FAS1, from the crystal structure of domains 3 and 4 [30], oriented so that domain 4 aligns with Fdp. For clarity, domain 4 is not shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
