Abstract
The Wrist and Radius Surgery Trial (WRIST) study group is a collaboration of 22 hand surgery centers in the US, Canada, and Singapore to showcase the interest and capability of hand surgeons to conduct a multicenter clinical trial. The WRIST study group was formed in response to the seminal systematic review by Margaliot et al. and the Cochrane report that indicated marked deficiency in the quality of evidence in the distal radius fracture literature. Since the initial description of this fracture by Colles in 1814, over 2,000 studies have been published on this subject, yet high level studies based on the principles of evidence-based medicine are lacking. As we continue to embrace evidence-based medicine to raise the quality of research, the lessons learned during the organization and conduct of WRIST can serve as a template for others contemplating similar efforts. This paper will trace the course of WRIST by sharing the triumphs, and more importantly the struggles, faced in the first year of this study.
Keywords: distal radius fracture, randomized clinical trials, multicenter trials
What is WRIST?
Distal radius fractures (DRFs) are the second most common fractures, after hip fractures, sustained by elderly Americans. (1) Traditionally, DRFs in the elderly population have been treated with casting, with acceptable, although not ideal, radiological and functional results. (2–5)Surgical treatment has become more widely used, but there have been a scarcity of high level evidence studies to support which, if any, of the surgical treatments provide better results. Also, do the results after surgical fixation differ from those following casting? (6–9) The American Academy of Orthopaedic Surgeons guidelines on the treatment of distal radius fractures do not recommend for or against volar locking plate use in patients over the age of 55, labeling the evidence “inconclusive.” (10) This lack of evidence arises from the paucity of large scale randomized clinical trials conducted, which are the cornerstone of evidence-based medicine. Recently Arora et al. randomized Austrian participants to receive cast immobilization or internal fixation with volar locking plates. (2) Outcomes were pain, wrist range of motion, Disabilities of the Arm, Shoulder, and Hand score, and Patient-Rated Wrist Evaluation score. They found no statistically significant differences between participants who received casting and those who received internal fixation. (2) This trial, however, limited its outcomes to only upper extremity functional assessment. DRFs may affect patients’ overall quality of life, and these outcomes may be more relevant to participants than specific upper extremity function. Furthermore, the study was performed at 1 site, which limits general applicability. The Wrist and Radius Injury Surgical Trial (WRIST) was created to answer questions about treatment of DRFs in elderly patients via the largest and most complex trial the study PI (KCC) had undertaken. In fact, WRIST is probably the largest multicenter international randomized trial undertaken in hand surgery. The goal of WRIST was to randomize participants 60 years of age or older to 1 of 3 surgical procedures (percutaneous pinning, external fixation with or without pinning, and internal fixation with volar locking plates). Participants who opted not to have surgical fixation would also be followed as a control group. Approximately 3 years before participant recruitment commenced, a consensus meeting was held to distill the study questions. The participating hand surgeons wished for WRIST to be the most comprehensive study of DRFs in the elderly. To this end percutaneous pin fixation and external fixation were include in the randomization scheme, despite these 2 treatments being used for only 10% of Medicare beneficiaries. (11, 12) This study is designed to provide strong evidence regarding the value of volar plating technology through rigorous clinical trial design. The choice to leave casting out of the randomization scheme was made during the consensus meeting. All participating hand surgeons agreed that randomizing patients into a nonsurgical treatment would be problematic due to the predictable loss of reduction that quite often results. The panel also recognized the need to assess nonoperative patients who declined surgery and made the decision to statistically adjust the outcomes between the surgical and nonsurgical groups.
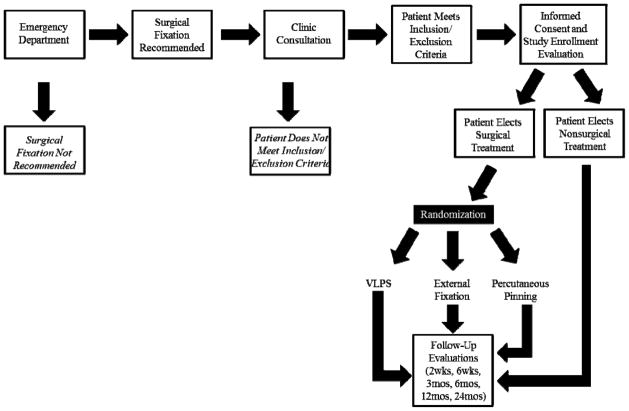
After enrollment, participants are to be followed for 2 years, and outcome measures include the Michigan Hand Outcomes questionnaire, quality of life assessment, and functional and radiological measures. Figure 1 details the flow of study participants. WRIST was conceived in the spring of 2007 at the University of Michigan. Originally comprised of 4 sites, the project has expanded over 5 years to include 22 sites in 3 countries and 11 US states and is jointly funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging. WRIST was given full approval by the National Institute of Health (NIH) in January 2012. Participant recruitment started in April 2012. Figure 2 details the steps involved in planning and executing WRIST from its 2007 inception through the end of 2012.
Figure 1.
Flow of WRIST participants from presentation to emergency department to completion of follow-up visits.
Figure 2.
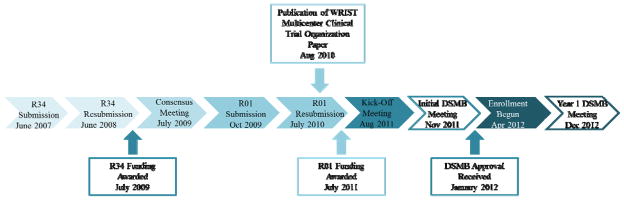
WRIST planning and execution.
The purpose of this paper is to share the experiences of the WRIST Study Team, including the many challenges we faced and the solutions we instituted to achieve a successful completion of this study.
Randomized Clinical Trials: A brief review
At its simplest, a randomized clinical trial involves the allocation of participants into 2 or more groups using a randomized list. The random assignment ensures the least amount of bias, which is a common occurrence in observational studies. (13) The Checklist to Evaluate a Report of Non-Pharmacological Trial lists the reporting requirements for randomized trials like those conducted in hand surgery. (14) Because these items should be reported, the list can also serve as a checklist of items to consider when planning a trial. (Table 1) When planning a multisite trial, the most important component is ensuring that participant experiences, from enrollment to intervention to follow-up assessment, are identical at every site. Alterations in any of these elements can introduce bias into the results.
Table 1.
Checklist of items to consider when planning a multisite randomized clinical trial*
| Randomization and Blinding |
| Are randomization schemes appropriate for the type of trial and the desired outcome? |
| Are randomization assignments blinded, when appropriate? |
| Are assessors blinded, when appropriate? |
| Intervention |
| Has the intervention and study procedures been standardized across sites? |
| Are plans in place to ensure that sites are adhering to the standard? |
| Participant Follow-up |
| Is participant follow-up standard across sites? |
| Are plans in place to assess each site’s follow-up rate? |
| Are plans in place to deal with missing data? |
Adapted from The Checklist to Evaluate a Report of Non-Pharmacological Trial (CLEAR NPT)
Major hurdles in the WRIST journey
Regulatory issues
Many studies are overseen by a data safety and monitoring board (DSMB) in addition to an institutional review board (IRB). A DSMB is an independent board appointed by the NIH that reviews study progress and adverse events, performs interim analysis, and makes recommendations to the funding agencies about the study conduct. (15) In WRIST, the coordinating center had to revise the proposal and include additional documents according to DSMB recommendations prior to the IRB approval.
Every human subjects study requires IRB approval at each site before the start of recruitment. It is an important aspect in every project and depends upon the development of a stringent Manual of Operations and Procedures (MOOP). In WRIST, the MOOP provides instructions on how to perform all facets of the study including participant randomization, operative procedures, schedule of follow-up visits, and data entry, and it also includes copies of all study forms. The MOOP serves as reference guide to the IRB, investigators, and research staff. The study team needs to anticipate the necessary time and work required for obtaining IRB approval. There are, however, many aspects that are outside the investigators’ control, such as individual IRB boards’ fixed schedules for review and the multiple amendments that are often required to satisfy the queries of the IRB reviewers. Required changes may include revisions to the protocol and other study documents. Because the study protocol must be identical across all sites, changes required at the coordinating center can lead to substantial setbacks for other sites as well. As a general rule, at least 6 months should be allotted for completion of the IRB approval process, although it can take much longer. (15, 16) In our experience it took some sites nearly a year to obtain approval.
In WRIST, every one of the aforementioned barriers was encountered. It took longer than anticipated to receive final approval at the coordinating center, the University of Michigan, owing to multiple modifications to the protocol and MOOP. Every change required approval by all 22 of the participating sites’ IRB or ethics committees. A site’s ability to initiate an application was contingent on the coordinating center’s IRB reaching final opinion on all study items. There were several concerns regarding randomizing patients that had to be addressed at various participating sites. Such processes that delay the final approval are invariably experienced by most large multicenter trials. (16–18) Regulatory authorities had concerns regarding ensuring uniformity of study conduct across all sites because of the involvement of 22 sites, some of which are located internationally. In addition, queries about study eligibility criteria and randomization of elderly study participants aged 60 years and above were unique to this study. As a randomized control trial, WRIST faces different challenges from a prospective trial because of predetermined strict eligibility criteria that were agreed upon by the panel of experts and approved by NIH review committees to accomplish the study’s specific aims. This limited the number of eligible patients in our screening pool. Finally, the 24-month follow-up visit was not standard of care, meaning that processes needed to be in place at all sites to ensure that neither the patient nor their insurance payer were burdened. Despite having funding, a completed protocol, and all the required study documents, WRIST could not begin screening patients before obtaining DSMB and IRB approval to do so. To attain our projected goal of required number of participants in the allocated time, WRIST team included sites from within and outside the United States. This turned out to be even more challenging because of differences in regulatory requirements, approval procedures, and research conduct specific to those countries. Such challenges are a common occurrence in multicenter international trials. (17) In the end, WRIST overcame these hurdles, and all the sites now have regulatory approval.
Another crucial aspect was developing and executing a contract between the coordinating center and sites. This document covered financial issues, such as budget and funds release, as well other issues including authorship of publications and ownership of collected data. Contracting is another trial aspect that is outside of the investigators’ control. Typically the grants and contracts specialists at each institution are responsible for reviewing and modifying the contract and when the 2 parties have reached an agreement on those terms, the contract will be executed. This process can be delayed for a variety of reasons. In WRIST, as in other large trials, contract distribution was held up at the coordinating center because of an unusually large number of contracts that needed to be processed through one contracting department. Contracts were also impeded at the sites because of similar high volume of contract requests. Despite the delays in this process, contracts have been executed at all sites.
Participant enrollment and randomization
After a laborious effort in procuring IRB approval and executing contracts, sites were able to initiate screening for eligible patients. Achieving the required sample size is crucial for a clinical trial to have adequate power to test the study hypotheses. However, WRIST found many fewer eligible patients than expected. This is not uncommon in clinical trials. (19–21) Only 30% of presenting patients aged 60 year and older with a DRF were eligible, and nearly 50% of eligible patients we approached declined participation. Age, sex, and race are recorded for all screened participants to monitor any marked differences between patients who choose to enroll and those who do not. Subject recruitment for randomized clinical trials is difficult because many patients, as well as their families, are uncomfortable with randomization. WRIST had refusal from eligible patients upon the advice of their family or surgeon friends with the fear of not getting assigned to the treatment of their choice. Despite a lack of strong evidence to support its effectiveness over other techniques, the popularity of the volar plating procedure hinders many eligible subjects from considering the other alternatives such as external fixation or pin fixation that has been in use for years.
Increased screening could alleviate some of the impact of many ineligible patients, but the raw number of DRFs encountered by participating surgeons has been much lower than expected. Weather may have had an impact on this. We began recruitment in the spring and summer months. A cursory analysis of 100% Medicare data for 2007, obtained as part of a previous project, (12, 22, 23) shows that the winter months experience a 47% increase in DRF incidence over the spring and summer months. Elderly DRF incidence is at its lowest in April and its highest in January. Adverse weather conditions and slippery surfaces due to snow, ice, and freezing rain in winter are environmental risk factors associated with increased DRF incidence in elderly population. (24–28) Many WRIST sites are in climates that see adverse winter weather; with the arrival of the winter season, we expect to have a greater number of patients to screen. Furthermore, one of the solutions we have instituted is to recruit more Canadian sites into this study, where winters are harsh. Discussion with hand surgeons in Canada indicates a potentially large number of eligible subjects during the winter months. In Canada, often the least expensive treatment that yields acceptable outcome is applied because of the national healthcare system. The belief by patients and surgeons for the merit a particular treatment technology is less entrenched than in the US.
Process challenges in the WRIST endeavor
Identification of appropriate and committed sites
One of the most important aspects of conducting a successful multicenter study is the identification and participation of committed sites that collaborate continuously and have ready access to eligible patients. (15) It is crucial to have commitment from the coordinating center to perform its functions and fulfill its role in the best interest of the whole group and to have commitment from the participating sites to perform and meet their individual goals. (29) Collaboration and cohesion between the lead investigators at the sites and their whole team comprising of other investigators, fellows, residents, and research staff is necessary for a multicenter study to be successful. (29)
Sites in all regions of the US and Canada were approached to participate in WRIST. Our goal was to select sites that would provide a diverse participant population. We also wanted to include a variety of practice settings: small and large, rural and urban, and academic and community. Participating in WRIST requires a dedicated principal investigator and staff. The WRIST study has the advantage of such committed sites’ participation. Unfortunately, some sites have dropped out of the project because of difficulty in following the required rigorous requirements. The WRIST group was able to replace these sites with several new sites that are as capable and committed as the other participating sites. Regional and site-related variables are included in the planned statistical analysis to determine if these factors play any role in outcomes of DRFs in the elderly.
Complicated process to get access to data entry
A standardized data entry process is mandatory in a multicenter study to ensure data quality and integrity across all sites. Data entry and transfer processes need to be conceived, tested, and executed before participant recruitment. WRIST has a secured, web-based data capture application called Research Electronic Data Capture (REDCap), developed at Vanderbilt University. The application and database servers used for REDCap at the University of Michigan are located on an internal network for increased security. Outside users, in this case personnel at the participating sites, are required to access the system remotely by logging into the University of Michigan environment via a secure virtual processing network. This necessitates 2 different passwords processed by Michigan Institute for Clinical Health and Research and the REDCap team. Passwords are conveyed to the coordinating center which in turn verbally relays them to the sites. WRIST sites have encountered several problems due to inability to download the required virtual processing network to access REDCap and passwords being lost or forgotten and having to be reset. With experience, this process issue is solved, and all sites are now able to access REDCap and enter data with ease.
Transmission of radiological images to the coordinating center
Participating sites have agreed to send the de-identified x-rays of study subjects to the coordinating center to confirm their eligibility and to track the progress of treatment. The coordinating center used the free file transfer application, Dropbox, to set up a secure online location where the sites can place their images directly. However, the ability to read the images was an issue because the scale was not consistent from site to site, meaning that angles were measured accurately but distance measurements were not. After extensive discussions with the radiology department at the central coordination site, we found a solution to this issue. The images placed in Dropbox in the Digital Imaging and Communications in Medicine format has inbuilt measuring tools with which the faculty can access and read the images from sites accurately. Table 2 provides a list of issues commonly encountered in randomized multicenter clinical trials and some potential solutions.
Table 2.
Issues in randomized multicenter clinical trials and solutions
| Issue | Solution |
|---|---|
| Participant issues | |
| Patient preference for a particular treatment. Apprehensive to treatment choice by random allocation.14 |
Educate and assure patients that none of the treatment options is significantly worse. Try to assuage fear about randomization by thoroughly explaining aspects of each treatment. |
| Barriers (age, socioeconomic status, personal temperament, language or culture) | Targeted approaches through telephone calls, emails, fliers or personal modes such as direct interaction with potential participants. |
| Informed Consent-complex and difficult to understand language, lengthy details about the study | Consent process should be made as simple and easy as possible. Brief consent document that covers only necessary information about the study with easy language especially in elderly population.a |
| Access Transportation to and from research site. Lack of private or public transportation especially in elderly.a |
Provide transportation, cover the cost of travel, parking vouchers, and financial incentives to participants. Engage family members, friends, and local support groups of the participant to provide transportation for follow-up appointments. |
| Fewer eligible than expected, smaller percentage agreeing to participate, recruitment targets too high.14 | Perform pilot studies to make proper assessments of eligible, acceptance or rejection rates of participation in study |
| Poor recruitment numbers | Increase the number of participating centers (most effective, feasible, and ethical strategy)b Inclusion of centers with track records of high recruitment in trials as they have dedicated people with required experience.a |
| Time | Reassurance of offering follow-up at weekend, afterhours, according to patient convenience.b |
| Ethnic barriers-ethnic differences between researchers and potential participants.a | Personal recruitment approaches, educating subjects about the goals of the study and involving bilingual, bicultural researchers when possible. |
| Fear of complications, side effects, or lack of control over study especially in elderly. | Reassurance, patient education and effective communication by researchers. |
| Family member, friends discouraging from participating in a study owing to the time commitment, travel required, and no additional benefit seen. | Educate family member about risks and benefits of participating in study. Inform family members to convey the importance of research (benefit to others rather than individual benefit) that results in no harm to the particular individual by participation in the study. |
| Recruitment issues-patient refuses to participate in a study. | Prepare the patient to receive information from the researcher before actual explanation of the research study. Be aware of patient’s comorbidities, special needs, and their past medical history.c Establish good rapport through effective communication. Offer concise explanation and overview of the research project. Inform patients at the beginning that participation is voluntary, can withdraw anytime, and their healthcare will not be compromised at any time based on their decision. Give the patient time to think deeply about the study and discuss with family members, relatives, and friends to help patients arrive at a decision. |
| Comorbidities and mortality in elderly in preventing recruitment.a | Inclusion and exclusion criteria should be carefully established so as not to exclude all elderly. Broaden the inclusion criteria to allow such participants without compromising the study objectives. Appropriate changes to sample size and lengthy enrollment period may be needed in studies involving elderly. |
| Investigator Issues | |
| Time constraints | Involve in one trial at a time allowing maximum time and dedication. |
| Lack of experienced staff | Employ team who are committed, have research experience, and train them adequately according to the study protocol. |
| Research staff schedule | Flexibility in time and days to perform participant follow- up. |
| Trial Design issues | |
| Complex trial design | Simple design that accommodates all the study objectives. |
| Lack of sufficient funding | Allot funds wisely and employ measures to cut costs where possible. Explore cost-effective strategies to conduct the study. |
| Expert team | Include experienced investigators and research staff and train them as applicable for the trial being conducted. Include multidisciplinary teams. |
| Data Issues | |
| Study design, data management and data analysis issues | Involve a statistician and/or an epidemiologist from the beginning of the study. They should be committed and work closely with the investigators. |
| Data entry and transfer issues | Develop and test a standardized process before the beginning of the study. |
| Participating Site Issues | |
| Keeping teams motivated in a long multicenter trial | Frequent face-to-face meetings with all participating sites dedicated for research study or as an adjuvant to national or regional scientific conferences. Regular conference calls with status updates and proposal of new strategies for efficient conduct of the study. |
| Conflicting issues between sites | Consensus reached through exchange of ideas and problem solving. Develop study protocol that is acceptable to all participants without compromising the study objectives. |
| All the participating sites do not perform equally | Identify problem sites and resolve the issues. Include sites with ability, experience, and commitment to perform well for the benefit of the study. |
| Location of meetings | At the coordinating center or at a location that is easily accessible to majority of the participants |
| Publication issues | |
| Authorship and credit for writing | Specific acknowledgement of individuals who lead the study followed by acknowledgment to the whole study group. |
Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. Jan 22 2010;28(4):901–906.
Cook DJ, Blythe D, Rischbieth A, et al. Enrollment of intensive care unit patients into clinical studies: a trinational survey of researchers’ experiences, beliefs, and practices. Crit Care Med. Jul 2008;36(7):2100–2105.
Harris R, Dyson E. Recruitment of frail older people to research: lessons learnt through experience. J Adv Nurs. Dec 36(5):643–51.
Things that went in accordance with plan in WRIST
Online randomization
Eligible candidates who agree to participate in the WRIST study are randomized to 1 of the 3 treatment methods through Treatment Assignment Tool-University of Michigan. It is a web-based tool to obtain treatment allocation. Research coordinators were able to easily access the treatment assignment tool with passwords acquired at the initial setup. It not only replaced the traditional randomization envelope but also provided automatic continuous documentation of study treatment assignments.
Data entry
The issues with data entry through REDCap that occurred in the beginning of the WRIST study are now resolved. Research staff at all sites are able to access REDCap and enter data with ease. Each site’s data are double entered for the first 2 months after enrollment of the first participant, once by the research staff at the site and again by the research staff at the coordinating center. After the completion of initial data verification period, sites that demonstrate accuracy in data entry will send case report forms to the coordinating center for every tenth participant encounter for the continuous verification. Radiological images continue to be transmitted to the coordinating center at each visit.
People of WRIST
An important aspect of WRIST, without whom the study could not be conducted, are the people of WRIST. They include participating surgeons, research personnel, a statistician, an epidemiologist and many support staff. WRIST is empowered with such research teams who are committed and strive to screen all eligible patients, alert surgeons, talk to the participants, consent them, perform follow-up assessments, and communicate with the coordinating center to resolve any issues in the process. Other people such as physician assistants, medical assistants, surgery schedulers, nurses, and many others who provide care for the study subjects help the study to run smoothly. The WRIST team, especially the research staff, are a strength to this study as they drive the surgeons who are passionate about the study but busy with their professional commitments. Keeping the team motivated constantly with repeated emphasis on procedural matters and subject recruitment issues is vital in a large trial. (15) Regular conference calls are held among the research staff to discuss enrollment problems and strategies to improve the study conduct, including exchange of tips to help the entire team. A monthly WRIST newsletter addressing current issues, tracking the recruitment goals, announcements, and updates about the team members keeps the entire team well-informed of the study events. (Appendix 1)
Participant incentives
Study participants are the key players in a trial, and every step necessary to recruit and retain them in the study is therefore critical. Participants in WRIST receive $20 at each visit as an appreciation for their time and effort to fill out the questionnaires and help us with the conduct of study. IRB or ethics committees permit providing payment to subjects participating in research. (30) However, it is advisable to limit the amount given to serve as a compensation for a participant’s travel and loss of earnings rather than to induce or coerce their participation in the study. The coordinating center has a Human Subject Incentive Program that makes the payments to subjects upon receiving a request from research staff after each patient visit. The Human Subject Incentive Program is an easy, quick, and prompt method to deliver subject payments. Participants at sites are paid by the individual sites who invoice the coordinating center for reimbursement. There were no delays or any other issues regarding participant payments in the WRIST study.
Evidence-based medicine and comparative effectiveness studies are becoming de rigueur for an intervention to be widely accepted. The proper planning and conducting of randomized multicenter clinical trials is of utmost importance to support treatment decisions with strong, high level data. Sharing our experiences may help future conduct of multicenter clinical trials by streamlining solutions to the assortment of challenges that we faced in this study. We recommend allowing considerable time to clear all regulatory hurdles. Create a contingency plan to deal with low potential participant availability. Identify prospective sites to ensure they are committed and have the time and resources available. Develop a plan to refine recruitment and retention to maximize participation rate.
WRIST has been a test of resolve and patience in organizing and conducting a highly ambitious study. WRIST will strive to dispel many uncertainties in treating distal radius fractures by deriving evidence to guide treatment for this prevalent injury in older individuals.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute on Aging (R01 AR062066) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (To Dr. Kevin C. Chung).
WRIST Study Group: University of Michigan: Kevin C. Chung, MD, MS (Principal Investigator), H. Myra Kim, ScD (Biostatistician), Steven C. Haase, MD, Jeffrey Lawton, MD, Kagan Ozer, MD, Jennifer F. Waljee, MD, MPH, Kate Nellans, MD, MPH, Sunitha Malay, MPH, Melissa J. Shauver, MPH; Beaumont Health System: Rachel Rohde, MD (Principal Investigator), Kevin Baker, PhD; Beth Israel Deaconess Medical Center: Tamara D. Rozental, MD (Principal Investigator), Paul Appleton, MD, Edward Rodriguez, MD, Lindsay Herder, BA, Katiri Wagner; Brigham and Women’s Hospital: Philip Blazar, MD (Principal Investigator), Brandon Earp, MD, W. Emerson Floyd, BA; Duke University: Fraser Leversedge, MD (Principal Investigator), Katherine S. Pico, MD, Marc J. Richards, MD, David S. Ruch, MD, Suzanne Finley, EMT-P; HealthPartners Institute for Education and Research: Loree K. Kalliainen, MD (Principal Investigator), James W. Fletcher, MD, Cherrie A. Heinrich, MD, Christian M. Ward, MD, Brian W. Hill, MD; Kettering Health Network: Brent Bamberger, DO (Principal Investigator), Carla Robinson, PA-C, MPAS, Brandi Palmer, MS, PC, CCRP; Massachusetts General Hospital: David Ring, MD, PhD (Principal Investigator), Jesse B. Jupiter, MD, MA, Rajesh Reddy, BA; Mayo Clinic: David Dennison, MD (Principal Investigator), Sanjeev Kakar, MD, Alexander Shin, MD, Tyson Scrabeck, BS; Metro Health System: Harry Hoyen, MD, Kevin Malone, MD; National University of Singapore: Sandeep Sebastin, MCh (Principal Investigator), Poh Ling Tay; OrthoCarolina: Glenn Gaston, MD (Principal Investigator), Benjamin Connell, BA; Southern Illinois University: Michael Neumeister, MD (Principal Investigator), Nada N. Berry, MD, Reuben A. Bueno, Jr, MD, Mark McAndrew, MD, Jennifer Koechle, MPH; University of Connecticut: Jennifer Moriatis Wolf, MD (Principal Investigator), Craig M. Rodner, MD, Mark Cote; University of Manitoba: Tod Clark, MD, MSc, FRCSC (Principal Investigator); University of Oklahoma: Thomas Lehman, MD, PT (Principal Investigator), Kathy Carl, BA, CCRP; University of Pennsylvania: David Bozentka, MD (Principal Investigator), Scott Levin, MD, David Steinberg, MD, Annamarie Horan, PhD, Denise Knox; University of Rochester: Warren Hammert, MD, DDS (Principal Investigator), Allison W. McIntyre, MPH; University of Toronto: Brent Graham, MD, MSc (Principal Investigator), Christine Novak, PT, PhD; University of Washington: Jeffrey B. Friedrich, MD (Principal Investigator), Christopher H. Allan, MD, Douglas P. Hanel, MD, Jerry I. Huang, MD, Jason H. Ko, MD, Nicholas B. Vedder, MD, David Boman, Claudette L. Cooper; University of Western Ontario: Ruby Grewal, MD, MS (Principal Investigator), Joy MacDermid, PhD (Epidemiologist), Kate Kelly, M.Sc, MPH/Gero, Kristie Millman; Wake Forest University: Zhongyu Li, MD, PhD (Principal Investigator), Andrew Koman, MD, Beth Smith, PhD, Debra Bullard
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med 1989. 1989;149:2445–2448. [PubMed] [Google Scholar]
- 2.Arora R, Gabl M, Gschwentner M, Deml C, Krappinger D, Lutz M. A comparative study of clinical and radiologic outcomes of unstable colles type distal radius fractures in patients older than 70 years: nonoperative treatment versus volar locking plating. J Orthop Trauma. 2009 Apr;23(4):237–242. doi: 10.1097/BOT.0b013e31819b24e9. [DOI] [PubMed] [Google Scholar]
- 3.Synn AJ, Makhni EC, Makhni MC, Rozental TD, Day CS. Distal radius fractures in older patients: is anatomic reduction necessary? Clin Orthop Relat Res. 2009 Jun;467(6):1612–1620. doi: 10.1007/s11999-008-0660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect 2003. 2003;52:185–195. [PubMed] [Google Scholar]
- 5.Grewal R, MacDermid JC. The risk of adverse outcomes in extra-articular distal radius fractures is increased with malalignment in patients of all ages but mitigated in older patients. J Hand Surg [Am] 2007 Sep;32(7):962–970. doi: 10.1016/j.jhsa.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg. 2005 Nov;30A(6):1185–1199. doi: 10.1016/j.jhsa.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522. doi: 10.1002/14651858.CD006522.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handoll HH, Madhok R. Conservative interventions for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2003;(2):CD000314. doi: 10.1002/14651858.CD000314. [DOI] [PubMed] [Google Scholar]
- 9.Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080. doi: 10.1002/14651858.CD006080.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Surgeons AAoO. The treatment of distal radius fractures. Rosemont, IL: American Academy of Orthopaedic Surgeons (AAOS); 2009. [Google Scholar]
- 11.Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009 Aug;91(8):1868–1873. doi: 10.2106/JBJS.H.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KC, Shauver MJ, Yin H, Kim HM, Baser O, Birkmeyer JD. National variations in the utilization of internal fixation techniques for distal radius fractures in the United States Medicare population. J Bone Joint Surg. 2011;93:2154–2162. doi: 10.2106/JBJS.J.012802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung KC, Burns PB. A guide to planning and executing a surgical randomized controlled trial. J Hand Surg. 2008 Mar;33A(3):407–412. doi: 10.1016/j.jhsa.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Boutron I, Moher D, Tugwell P, et al. A checklist to evaluate a report of nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidem 2005. 2005;58:1233–1240. doi: 10.1016/j.jclinepi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Herrick LM, Locke GR, 3rd, Zinsmeister AR, Talley NJ. Challenges and lessons learned in conducting comparative-effectiveness trials. Am J Gastroenterol. 2012 May;107(5):644–649. doi: 10.1038/ajg.2011.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNay LA, Tavel JA, Oseekey K, McDermott CM, Mollerup D, Bebchuk JD. Regulatory approvals in a large multinational clinical trial: the ESPRIT experience. Control Clin Trials. 2002 Feb;23(1):59–66. doi: 10.1016/s0197-2456(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 17.Aban IB, Wolfe GI, Cutter GR, et al. The MGTX experience: challenges in planning and executing an international, multicenter clinical trial. J Neuroimmunol. 2008 Sep 15;201–202:80–84. doi: 10.1016/j.jneuroim.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duley L, Antman K, Arena J, et al. Specific barriers to the conduct of randomized trials. Clin Trials. 2008;5(1):40–48. doi: 10.1177/1740774507087704. [DOI] [PubMed] [Google Scholar]
- 19.Campbell MK, Snowdon C, Francis D, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007 Nov;11(4):iii, ix–105. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]
- 20.Kaur G, Smyth RL, Williamson P. Developing a survey of barriers and facilitators to recruitment in randomized controlled trials. Trials. 2012 Nov 21;13(1):218. doi: 10.1186/1745-6215-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997 Aug;18(4):328–352. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 22.Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011 Aug;36(8):1282–1287. doi: 10.1016/j.jhsa.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Chung KC, Shauver MJ, Yin H. The relationship between ASSH membership and the treatment of distal radius fracture in the United States Medicare population. J Hand Surg Am. 2011 Aug;36(8):1288–1293. doi: 10.1016/j.jhsa.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Oyen J, Rohde GE, Hochberg M, Johnsen V, Haugeberg G. Low-energy distal radius fractures in middle-aged and elderly women-seasonal variations, prevalence of osteoporosis, and associates with fractures. Osteoporos Int. 2010 Jul;21(7):1247–1255. doi: 10.1007/s00198-009-1065-0. [DOI] [PubMed] [Google Scholar]
- 25.Ralis ZA. Epidemic of fractures during period of snow and ice. Br Med J (Clin Res Ed) 1981 Feb 21;282(6264):603–605. [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson PW, Taylor J, Dawson A. The annual incidence and seasonal variation of fractures of the distal radius in men and women over 25 years in Dorset, UK. Injury 2004. 2004;35:462–466. doi: 10.1016/S0020-1383(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen SJ, Sargent DJ, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Contribution of weather to the seasonality of distal forearm fractures: a population-based study in Rochester, Minnesota. Osteoporos Int. 1999;9(3):254–259. doi: 10.1007/s001980050145. [DOI] [PubMed] [Google Scholar]
- 28.Flinkkila T, Sirnio K, Hippi M, et al. Epidemiology and seasonal variation of distal radius fractures in Oulu, Finland. Osteoporos Int. 2011 Aug;22(8):2307–2312. doi: 10.1007/s00198-010-1463-3. [DOI] [PubMed] [Google Scholar]
- 29.Hogg RJ. Trials and tribulations of multicenter studies. Lessons learned from the experiences of the Southwest Pediatric Nephrology Study Group (SPNSG) Pediatr Nephrol. 1991 May;5(3):348–351. doi: 10.1007/BF00867501. [DOI] [PubMed] [Google Scholar]
- 30.Dickert N, Emanuel E, Grady C. Paying research subjects: an analysis of current policies. Ann Intern Med. 2002 Mar 5;136(5):368–373. doi: 10.7326/0003-4819-136-5-200203050-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.