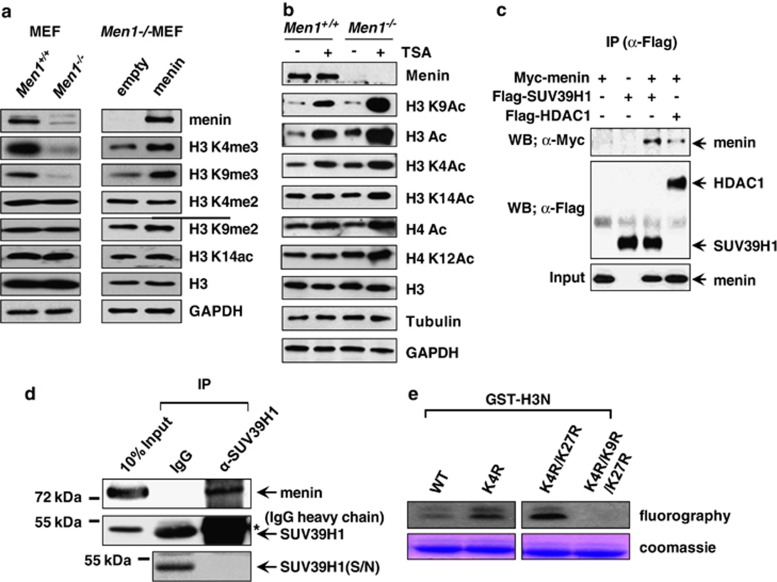
Figure 1.
Menin specifically associates with SUV39H1. (a) Whole-cell lysates of Men1+/+and Men1−/−MEFs were used for western blot analysis with antibodies against menin, H3K4me2, H3K4me3, H3K9me2, H3K9me3, H3K14ac, H3, and GAPDH (left panel). H3 and GAPDH served as loading controls. Men1−/− MEF cells were infected with control or menin-expressing retroviruses. Cell lysates from the infected cells were subjected to western blot analysis (right panel). (b) Menin's effect on histone covalent modification. MEF cells were treated with TSA (100 nℳ) for 24 h and whole-cell extract was prepared for western blot analysis. H3, Tubulin, and GAPDH are loading controls. (c) 293T cells were transfected with Flag-SUV39H1, Flag-HDAC1, and Myc-menin expression vectors as indicated. Whole-cell extracts were immunoprecipitated with anti-Flag antibody, and the immunoprecipitates were analyzed by western blotting using anti-Flag and anti-Myc antibodies. (d) Co-immunoprecipitation assay was performed to detect interaction between endogenous proteins in 293T cells. Anti-SUV39H1 antibody was used for immunoprecipitation. The asterisks * denote cross-reactive IgG heavy chain bands. As SUV39H1 co-migrates with and is masked by IgG heavy chain on SDS-PAGE, the supernatant fraction remained after IP is shown in parallel to show that SUV39H1 was efficiently precipitated by its antibody. (e) In vitro HMT activity associated with menin. HMT reaction was performed with menin immunoprecipitates and [3H]-SAM. Each reaction contains bacterially expressed GST-H3N (residues 1–57) wild-type (wt) or mutants (K4R, K4R/K27R and K4R/K9R/K27R) as indicated. Proteins were resolved on a SDS-polyacrylamide gel. The gel was stained with Coomassie (bottom) and exposed to film for fluorography (top)