FIGURE 4.
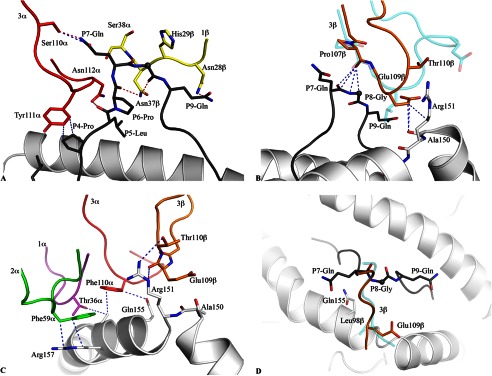
Contacts between the CA5 TCR and the HLA-B*35:08LPEP complex. A, CA5 TCR-peptide interactions were mediated primarily via the CDR3α (red) and CDR1β (yellow) loops. B, structural superposition of the CDR3β loops of the CA5 TCR (orange) and SB27 TCR (transparent cyan) in the corresponding ternary complexes. The conserved TCR-peptide interaction, with the peptide in black stick, is highlighted and the specific TCR-HLA interaction mediated by the CA5 TCR is compared with the SB27 TCR. C, conserved TCR-HLA interactions observed in the CA5 and SB27 ternary complex structures. D, top view of the CDR3β loop interaction with the HLA-B*35:08LPEP complex for the CA5 TCR (orange) superposed with the SB27 TCR (transparent cyan). This view shows the difference in orientation between the Gluβ109 and Leuβ98 side chains in the CA5 and SB27 TCRs, respectively. All structural representations follow the color scheme depicted in Fig. 3. Blue dashed lines represent van der Waals interactions; red dashed lines represent hydrogen-bond contacts; spheres represent the Cα atom of glycine residues.
