FIGURE 5.
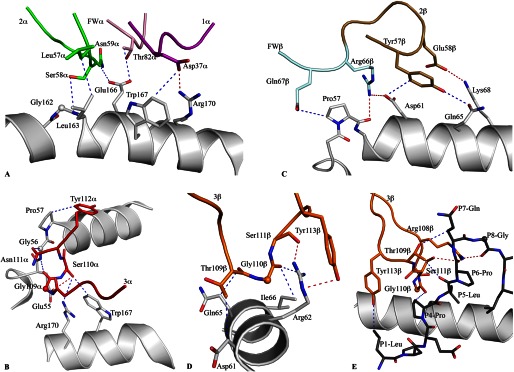
Contacts between the SB47 TCR and the HLA-B*35:08LPEP complex. A, the germline-encoded SB47 Vα chain, including CDR1α, CDR2α, and framework residues, interacted extensively with the α2-helix of HLA-B*35:08. B, the CDR3α loop bridged between the α1- and α2-helices, contacting a loop region from Glu55 to Pro57 of HLA-B*35:08. C, the CDR2β loop and its neighboring framework residues sat directly above the N-terminal part of the α1-helix of HLA-B*35:08, making contacts between Pro57 and Lys68. D, the CDR3β loop contacted a small stretch of the α1-helix of HLA-B*35:08, including Gln65. E, the SB47 TCR interacted with the N-terminal region of the peptide, including P1-Leu and P4-Pro to P8-Gly, exclusively via the CDR3β loop. All structural representations follow the color scheme depicted in Fig. 3. Blue dashed lines represent van der Waals interactions; red dashed lines represent hydrogen-bond contacts; spheres represent the Cα atom of glycine residues.
