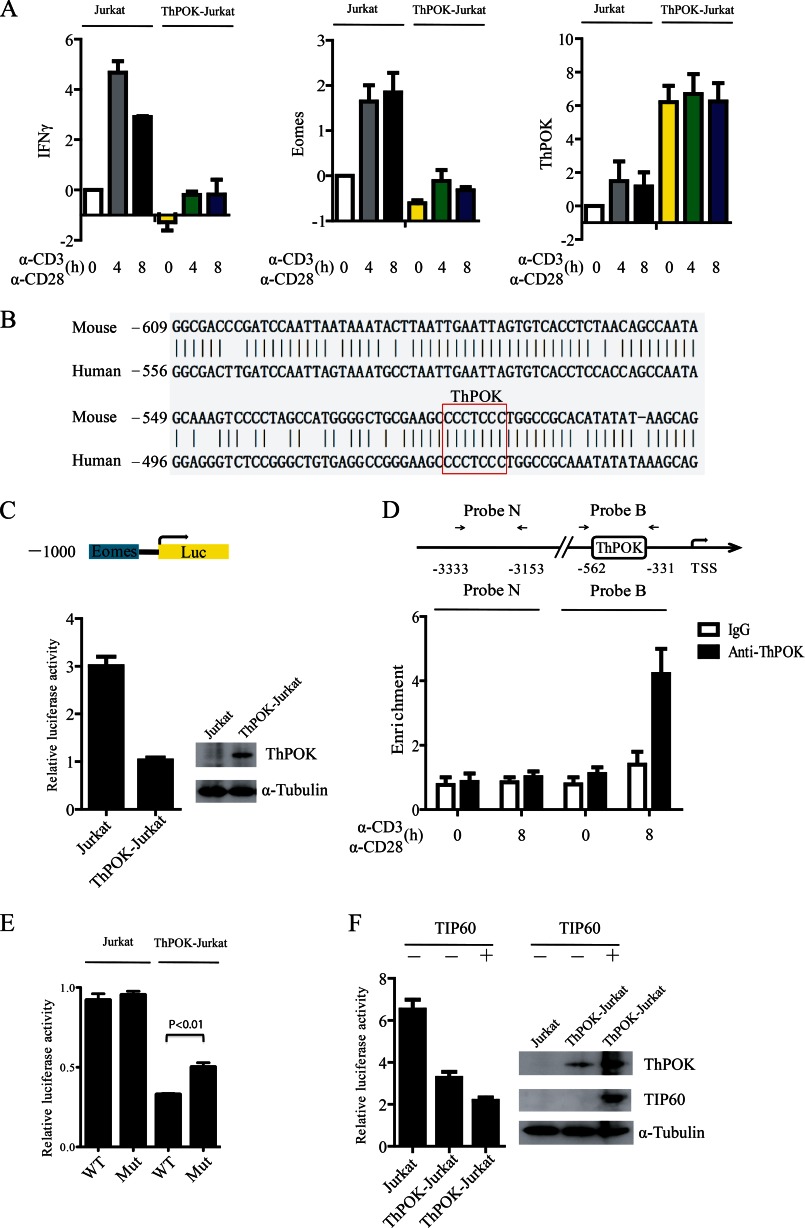
FIGURE 4.
Overexpression of TIP60 augments ThPOK-mediated repression of Eomes. A, a Jurkat cell line stably expressing ThPOK (ThPOK-Jurkat) was established. Jurkat and ThPOK-Jurkat cells were stimulated with α-CD3 and α-CD28 antibodies for the indicated time periods. Total RNA was extracted, and the mRNA levels of Eomes, IFNγ, and ThPOK were measured by qRT-PCR. Data show the mean of three separate experiments. Error bars indicate S.D. B, The 1-kb region (−1,000 to 0) of the Eomes promoter from mouse and human were aligned online using NCBI Blast (bl2seq). One consensus ThPOK binding site CCCTCCC was found on the Eomes promoter from both mouse and human. C, reporter transfection assay of the repressive activity of Eomes promoter by ThPOK in Jurkat and ThPOK-Jurkat cells. Cells were transfected with the pGL3-Eomes-Luc reporter which carries a 1,000-bp region of the Eomes promoter. 48 h later, luciferase activity was analyzed. D, CD4+ T cells were stimulated with anti-CD3/CD28 dynal beads for the indicated time periods. The binding of ThPOK to the Eomes promoter was determined by a ChIP assay with probe B, and probe N was used as a control. E, Jurkat or ThPOK-Jurkat cells were transfected with either pGL3-Eomes-Luc reporter or mutated reporter (CCCTCCC to CATTCCC) as indicated. The luciferase activity was analyzed 48 h later. Data show the mean of three separate experiments. Error bars indicate S.D. F, the pGL3-Eomes-Luc reporter was co-transfected with or without FLAG-TIP60 into Jurkat or ThPOK-Jurkat cells as indicated. The luciferase activity in the transfected cells was then determined. Error bars of C, D, and F show the S.D. from the mean in one experiment representative of three independent experiments.