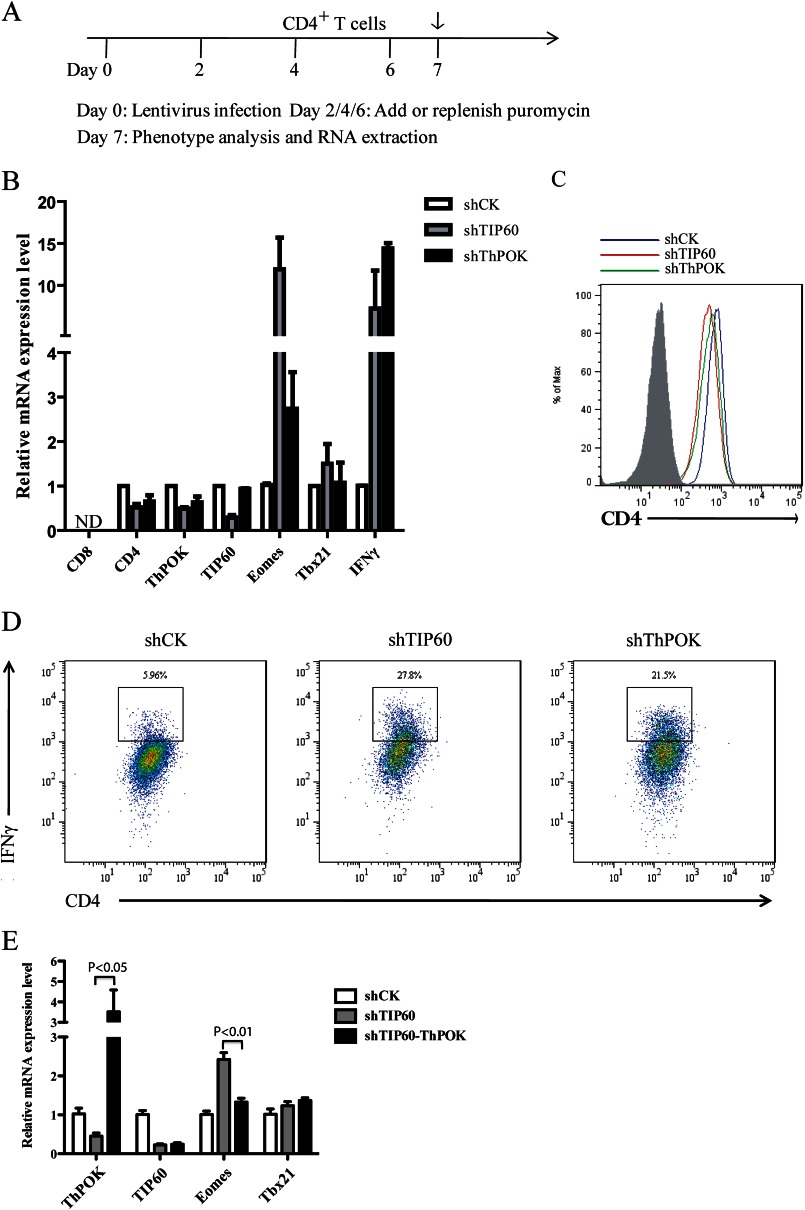
FIGURE 5.
TIP60 positively regulates ThPOK-mediated repression of Eomes in CD4+ T cells. A, timeline showing the knockdown of TIP60 and ThPOK in CD4+ T cells. On day 0, CD4+ T cells were transduced with lentivirus. 48 h later, cultures were treated with puromycin and replenished every other day. On day 7, selected CD4+ T cells were subjected to analysis by FACS or qRT-PCR. B, CD4+ T cells transduced with lentivirus-containing shRNA constructs targeting CK (control), TIP60, or ThPOK. Cells were cultured with anti-CD3/CD28 dynal beads and selected with puromycin for 5 days prior to RNA extraction. Total RNA was extracted, and mRNA levels of CD8, CD4, TIP60, ThPOK, Eomes, Tbx21, and IFNγ were determined by qRT-PCR. Data show the mean of three separate experiments. Error bars indicate S.D. C, expression of CD4 in selected CD4+ T cells described in B. Surface CD4 level was analyzed by flow cytometry. The shaded area represents the isotype control. D, IFNγ production by CD4+ T cells described in B. IFNγ level was observed after restimulation of cells with phorbol 12-myristate 13-acetate, ionomycin, and Golgi Stop for 4 h. E, CD4+ T cells transduced with lentivirus-containing shRNA constructs targeting CK (shCK) or TIP60 (shTIP60) as described in A. On day 4, shTIP60-transduced CD4+ T cells were also transduced with lentivirus-encoding ThPOK protein and GFP under the internal ribosome entry site. These cells (shTIP60-ThPOK) were cultured with anti-CD3/CD28 dynal beads and selected with puromycin for another 3 days. Both GFP+ and GFP− cells were selected by FACS prior to RNA extraction. Total RNA was extracted and mRNA levels of ThPOK, TIP60, Eomes, and Tbx21 were determined by qRT-PCR. Data show the mean of three separate experiments. Error bars indicate S.D.