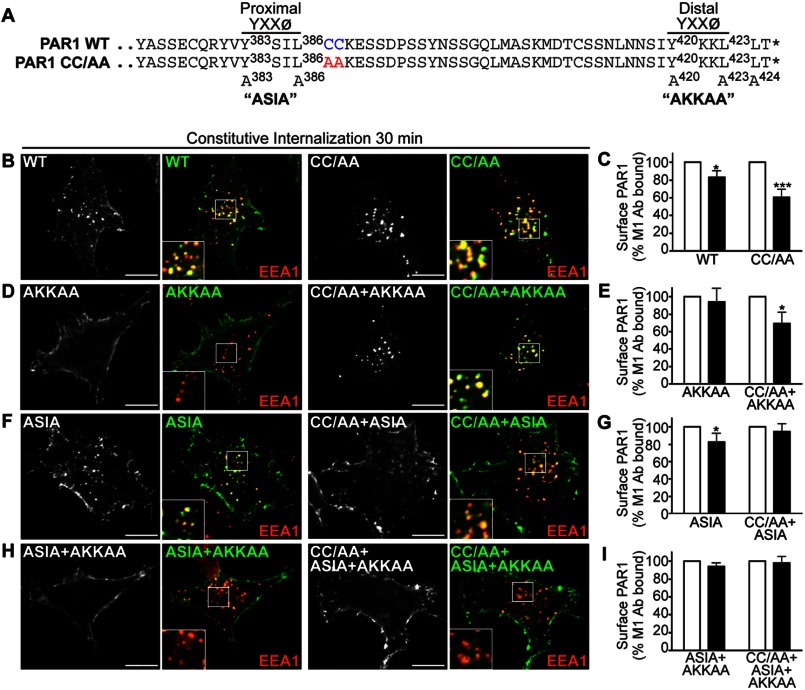
FIGURE 6.
Palmitoylation of PAR1 is important for proper utilization of C-tail tyrosine-based sorting motifs. A, alignment of PAR1 WT and CC/AA mutant C-tail amino acid residues is shown. The PAR1 C-tail tyrosine-based YXXØ motifs are shown, and superscript numbers indicate the position of the critical tyrosine and leucine residues that were mutated to alanine to generate ASIA and AKKAA mutants. The asterisk indicates the end of the protein sequence. B, D, F, and H, HeLa cells expressing FLAG-PAR1 WT and mutants were prelabeled with polyclonal anti-FLAG antibody, washed, and incubated for 30 min at 37 °C. Cells were fixed, permeabilized, and co-stained for EEA1, a marker of early endosomes, and imaged by confocal microscopy. PAR1 (green) and EEA1 (red) colocalization is revealed by the yellow color in the merged images. Insets are magnifications of the boxed areas. Images are representative of many cells examined in multiple independent experiments. Scale bar, 10 μm. C, E, G, and I, HeLa cells expressing FLAG-PAR1 WT or mutants prelabeled with M1 anti-FLAG antibody were incubated for 30 min at 37 °C. Cells were fixed, and the amount of remaining receptor-bound antibody was quantified by ELISA. The data (mean ± S.D.; n = 3) shown are represented as the percent of initial M1 antibody bound derived from three separate experiments performed in triplicate. The differences in constitutive internalization of PAR1 WT and various mutants compared with the 0-min control were significant (*, p < 0.05) or (***, p < 0.001) as determined by two-way ANOVA and Bonferroni post-hoc tests.