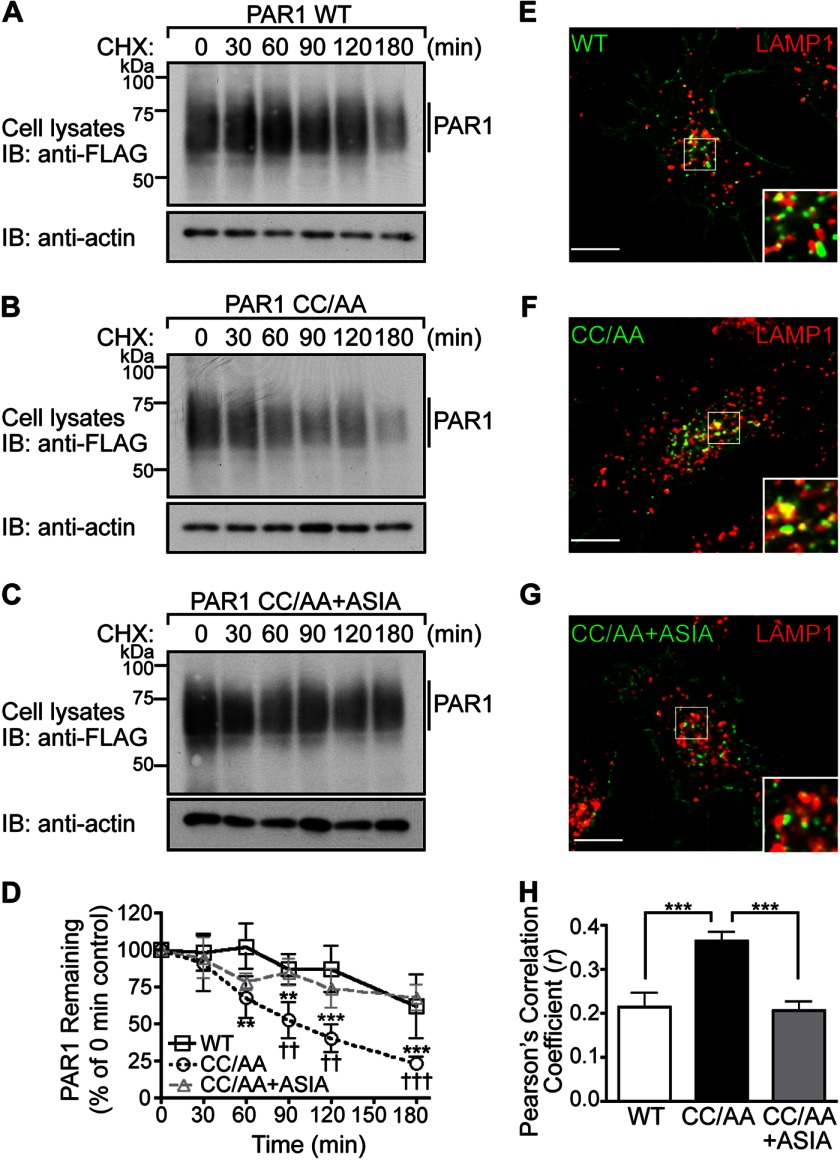
FIGURE 8.
The palmitoylation-deficient PAR1 mutant exhibits enhanced degradation. A–C, HeLa cells expressing FLAG-PAR1 WT, CC/AA, or CC/AA+ASIA mutants were incubated in the presence of 10 μm cycloheximide (CHX) for various times at 37 °C. Cells were lysed, and equivalent amounts of cell lysates were immunoblotted (IB) with polyclonal anti-FLAG antibody to detect PAR1 expression. Membranes were stripped and reprobed for actin. D, PAR1 WT and mutant degradation was quantified (mean ± S.D.; n = 3) and is expressed as the percentage of remaining PAR1 compared with 0 min control. The difference in the amount of PAR1 WT versus CC/AA was statistically significant (**, p < 0.01; ***, p < 0.001) as determined by two-way ANOVA and Bonferroni post-hoc tests. The difference in the amount of PAR1 CC/AA versus PAR1 CC/AA+ASIA remaining was statistically significant (††, p < 0.05; †††, p < 0.001) as determined by two-way ANOVA with Bonferroni post-hoc tests. E–G, HeLa cells expressing FLAG-PAR1 WT, CC/AA, or CC/AA+ASIA mutant were prelabeled with anti-FLAG antibody on ice for 1 h in the presence of 2 mm leupeptin. Cells were then incubated for 30 min at 37 °C, fixed, permeabilized, and costained for LAMP1, a marker of late endosomes, and imaged by confocal microscopy. The images are representative of many cells examined from three independent experiments. Scale bar, 10 μm. PAR1 (green) and LAMP1 (red) colocalization is revealed by the yellow color in the merged images. Insets are magnifications of the boxed areas. H, colocalization of PAR1 and LAMP1 was quantified by determining Pearson's correlation coefficients from seven different cells. The difference in PAR1 CC/AA versus WT and CC/AA+ASIA colocalization with LAMP1 was statistically significant (***, p < 0.001) as determined by one-way ANOVA with a Tukey post-hoc test.