FIGURE 1.
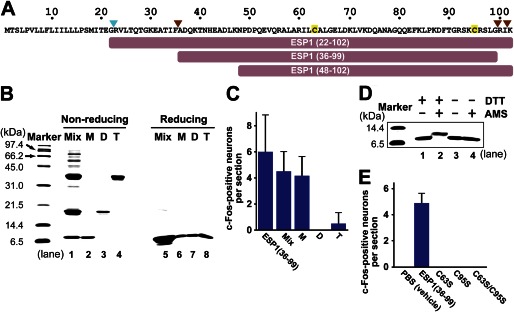
Characterization of ESP1 oligomerization and disulfide bridge formation. A, amino acid sequence of ESP1. The signal cleavage site is indicated by a cyan arrowhead, and the cleavage sites in the extraorbital lacrimal gland (9) are indicated by brown arrowheads. Cysteine residues are tinted yellow. The truncated constructs used in this study are shown as bars. B, SDS-PAGE analysis of the ESP1 oligomers under nonreducing and reducing conditions. The monomers (M), dimers (D), and tetramers (T) of ESP1 were purified to high homogeneity from a crude mixture of oligomers (Mix). Samples were subjected to an 18% SDS-PAGE under nonreducing (lanes 1–4) or reducing (lanes 5–8) conditions. C, analysis of the c-Fos-inducing activity of the ESP1 oligomers. The numbers of c-Fos-positive VNO neurons in BALB/c female mice stimulated by ESP1(36–99), Mix, M, D, or T were counted. The average values were obtained from slice sections of the mouse VNO (n = 6). Error bars represent S.D. D, analysis of intramolecular disulfide bridge formation using AMS. Preparations of the ESP1 monomers, either reduced with DTT (lanes 1 and 2) or nonreduced (lanes 3 and 4), were reacted with AMS (lanes 2 and 4). Samples were subjected to an 18% SDS-PAGE under nonreducing conditions. E, analysis of the c-Fos-inducing activity of the cysteine-to-serine mutants of ESP1. The numbers of c-Fos-positive VNO neurons in BALB/c female mice stimulated by PBS (vehicle), ESP1(36–99), or the mutant (C63S, C95S, or C63S/C95S) were counted. The average values were obtained from the mouse VNO (n = 6 slices, n = 3 mice).
