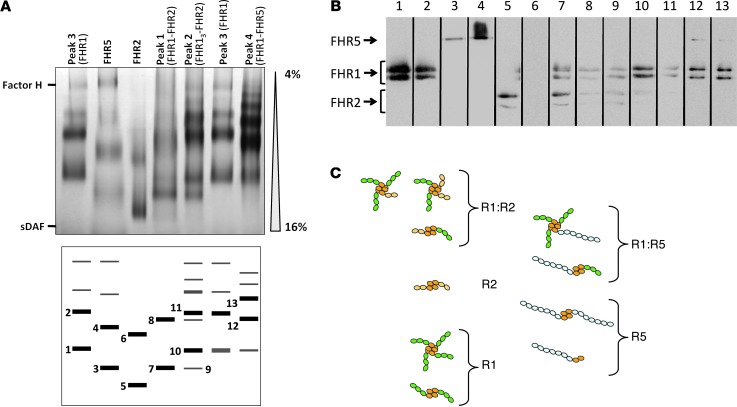
Figure 4. FHR1, FHR2, and FHR5 assemble into homo- and hetero-oligomers.
(A) Purified FHRs corresponding to the 4 FHR1-containing peaks from heparin chromatography, and FHR2 and FHR5 proteins purified from a FHR1-deficient individual, were analyzed in 4%–16% polyacrylamide native gels. sDAF (4 SCRs) and FH (20 SCRs) were used as molecular weight markers. Gels were silver stained. Protein complexes were obtained for each sample (bottom). (B) FHR composition of the numbered protein complexes in A was analyzed by SDS-PAGE. Comparison of the relative mobility of the bands obtained for each sample, together with the Western blot analysis, demonstrated the presence of different homo- and hetero-oligomeric complexes. For example, lanes 1, 4, and 5 were interpreted as FHR1, FHR5, and FHR2 homodimers, respectively. A faint and diffuse band (lane 3) running below the band of the FHR1 dimer could correspond to a FHR5 monomer. Lane 6 did not contain FHR2 (likely a protein contamination). Therefore, there was only 1 protein complex in plasma containing FHR2 (likely a dimer). Lane 7 was a FHR1-FHR2 heterodimer: it contained both FHR1 and FHR2 and presented mobility intermediate between the FHR2 and FHR1 homodimers. Lanes 12 and 13 corresponded to different FHR1-FHR5 hetero-oligomers. Lanes were run on the same gel but were noncontiguous (black lines). (C) Putative structure of these complexes, based on structural data demonstrating that the first 2 N-terminal SCRs (orange) of these proteins formed dimers in a head-to-tail orientation (13).