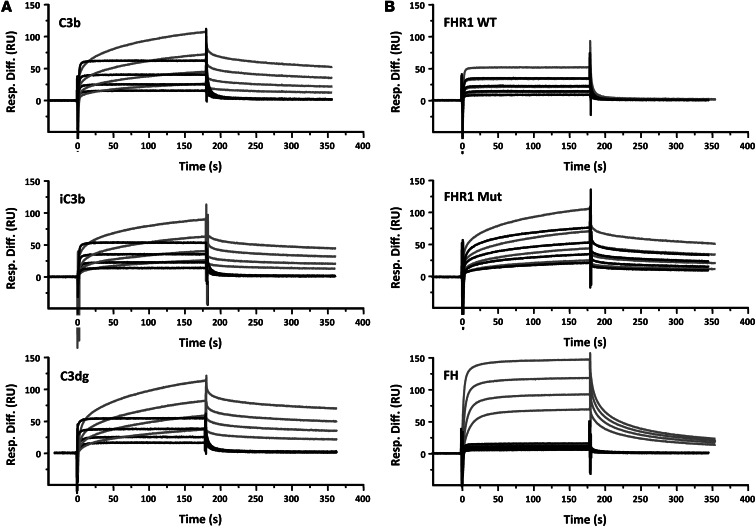
Figure 7. Mutant FHR1 shows increased binding to surface-bound C3b, iC3b, and C3dg.
(A) SPR analysis of native (black lines) and mutant (gray lines) FHR1 binding to C3b, iC3b, and C3dg. C3b (150 RU) was amine-coupled to a CM5 Biacore chip and used as a nidus for convertase formation. Further C3b (1,240 RU) was deposited on the chip surface by flowing FB and FD to form C3bBb, followed by C3 as convertase substrate. The surface C3b was converted to iC3b by incubating with FH and FI until the surface no longer supported convertase formation. Conversion to C3dg was achieved by incubating the iC3b surface with soluble CR1 and FI until no further C3c was released. 1:2 serial dilutions from 28 μg/ml native and mutant FHR1 were flowed across the surface for 3 minutes, then allowed to dissociate for 3 minutes prior to regenerating the surface. Avidity effects affecting dissociation of mutant FHR1 are clear. (B) Comparative binding of native and mutant FHR1 and FH to C3b and iC3b. Serial dilutions of native FHR1 (WT; 28 μg/ml), mutant FHR1 (Mut; 28 μg/ml), and FH (bottom; 26 μg/ml) were flowed across the C3b surface (gray lines) as described in A. The surface C3b was converted to iC3b by incubating with FH and FI until the surface no longer supported convertase formation. Native and mutant FHR1 as well as FH were flowed across iC3b (black lines) at identical concentrations as in A. Binding of native or mutant FHR1 was little affected by conversion of C3b to iC3b, whereas binding of FH was almost eliminated.