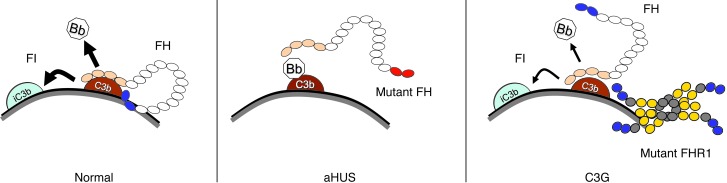
Figure 9. Proposed model for a novel pathogenic mechanism in C3G.
FH is an elongated glycoprotein of 155 kDa composed of 20 SCRs (small circles). FH presents C3b binding sites at each end of the molecule. The N-terminal C3b binding site mediates the accelerated decay of the AP C3 convertase (C3bBb) and the cofactor activity for the FI-dependent proteolytic inactivation of C3b. The C-terminal region binds both C3b and polyanions normally present in the cell surfaces (e.g., sialic acid, heparan sulfates, and glycosaminoglycans). This region is essential for the complement regulatory activity of FH on surfaces and to discriminate between self and pathogens, which normally lack these polyanions on their surfaces. Extensive experimental data generated during the last 10 years has provided conclusive evidence that mutations disrupting the functional activity of the C-terminal region, like those associated with aHUS, decrease the avidity of FH for cell surfaces and impair complement regulation (34–36). The data reported here suggest similarities between the established model for the aHUS-associated FH mutations and the FHR1 mutant described here. We therefore propose that multimerization of the FHRs as a consequence of duplication of the oligomerization domain in mutant FHR1, FHR2, or FHR5 proteins increases binding to surface-bound C3b, iC3b, C3dg, and carbohydrates, resulting in enhanced competition with FH that decreases its complement regulatory capacity and causes different degrees of cell surface complement dysregulation.