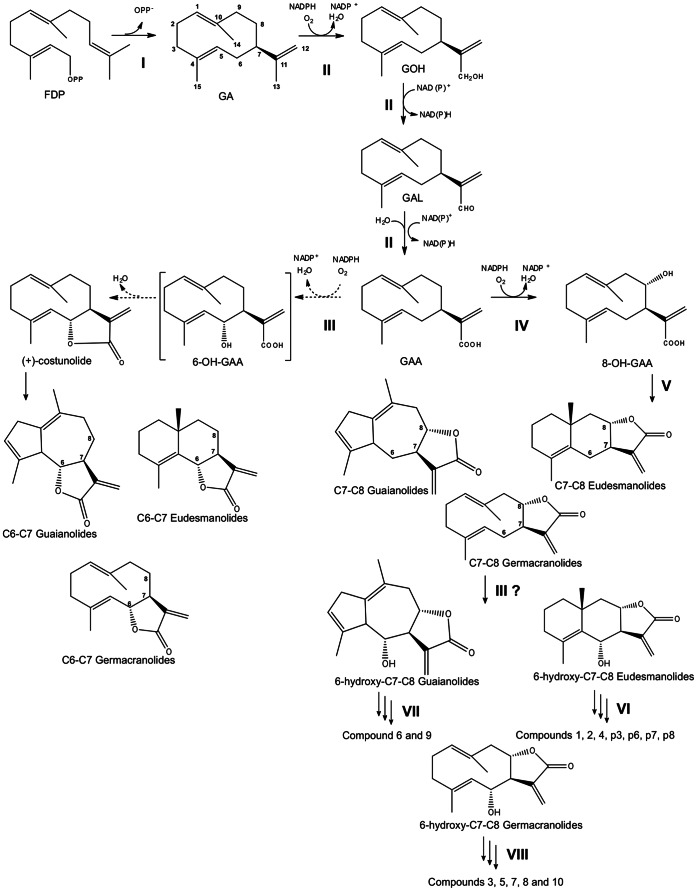
Figure 2. Proposed biosynthetic routes to C6–C7 and C7–C8 sesquiterpene lactones.
I, Cyclization of farnesyl diphosphate (FPP) to (+)− germacrene A (GA) by (+)− germacrene A synthase (TcGAS). II, Oxidation of GA via germacra-1(10),4,11(13)-trien-12-ol (GOH) and germacra-1(10),4,11(13)-trien-12-al (GAL) into germacra-1(10),4,11(13)-trien-12-oic acid (GAA) catalyzed by an NADPH-dependent single P450 enzyme (TcGAO). III, Hydroxylation at the C6 position of GAA or of various C7–C8 lactones by a second P450 enzyme (TcCOS). IV, Hydroxylation at the C8 position of GAA, and V, subsequent spontaneous lactonization. VI, VII, and VII involve extra oxidative steps and esterification with glycosyl, tigloyl and acyl groups, and cyclization of the sesquiterpene backbone to get to the structures in Figure 3 and 4.