Abstract
Purpose
To investigate ocular dimensions in African-Americans with the long anterior zonule (LAZ) trait.
Methods
Sixty-one African-American LAZ subjects and 61 age, race, and gender-matched controls were compared relative to central corneal thickness (CCT), central corneal curvature (CCC), axial length (AL), and subjective refraction (SR).
Results
LAZ right eyes had a mean SR = +1.75 D ± 1.82 D and were 1.58 D (95% CI=0.83 to 2.31 D, P<0.0001) more hyperopic on average than control right eyes. LAZ right eyes also had an AL that was 0.69 mm (95% CI=0.34 to 1.04 mm, P<0.001) shorter on average than control right eyes. Similar results were found for left eyes. No differences were found relative to CCC and CCT (P>0.05).
Conclusions
LAZ eyes in this dataset tended to be more hyperopic and had axial lengths that were shorter than control eyes, characteristics that are consistent with elevated risk for angle-closure glaucoma.
Introduction
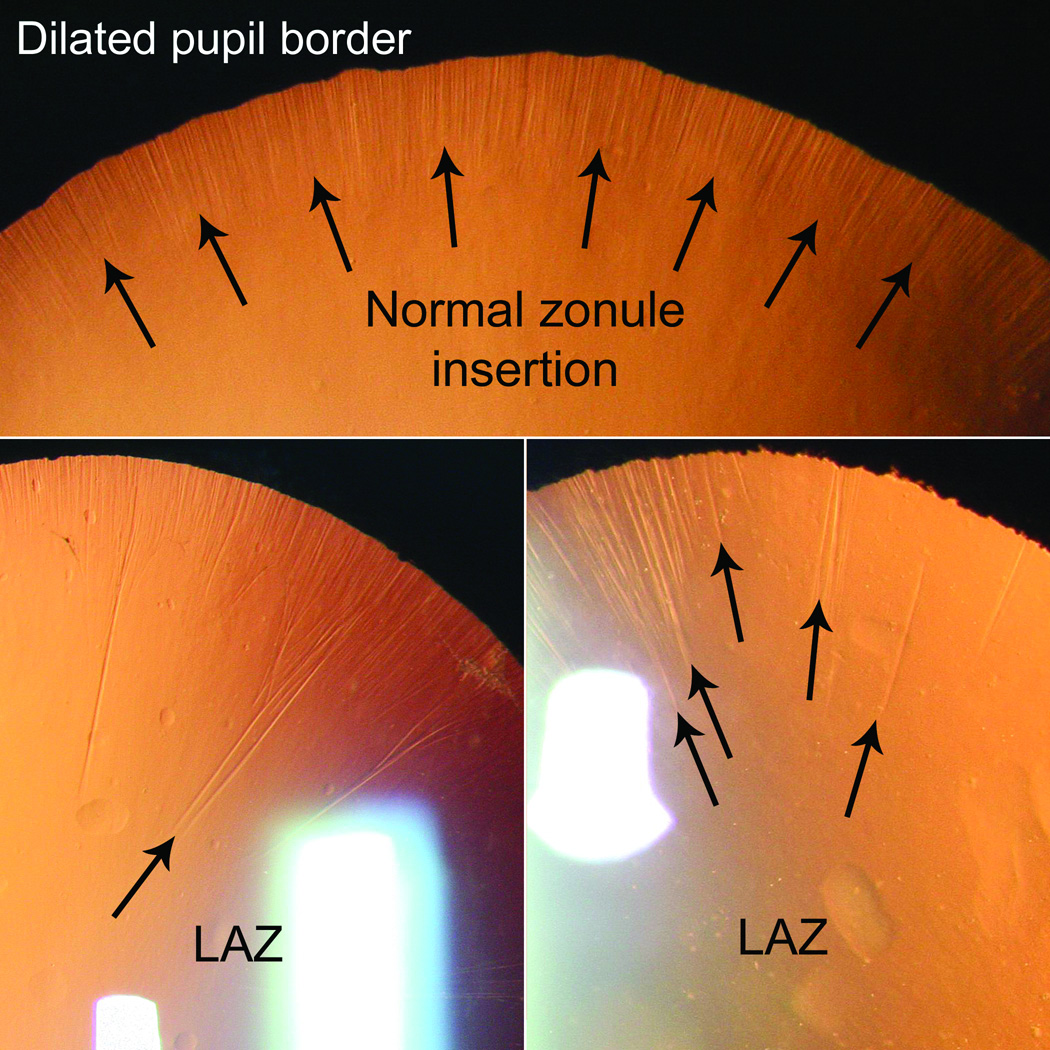
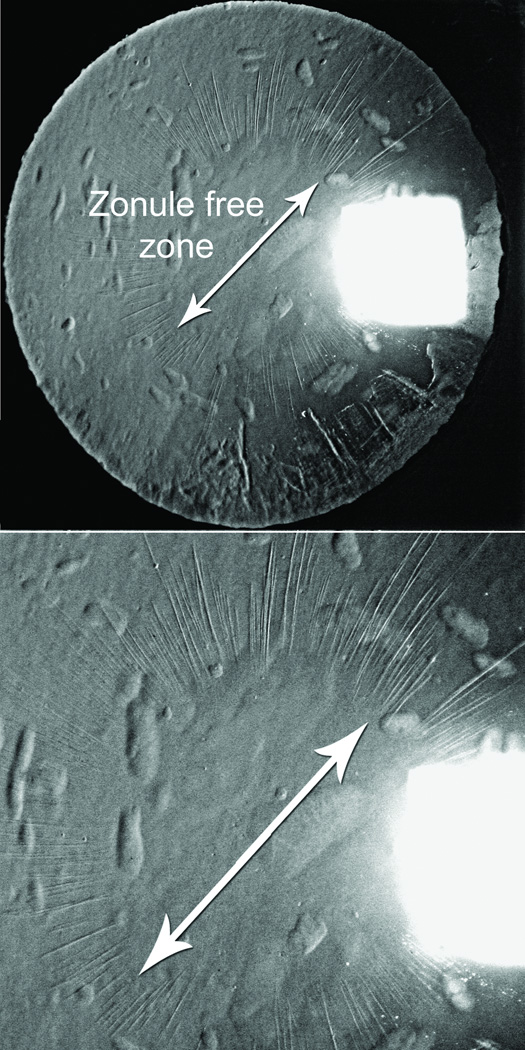
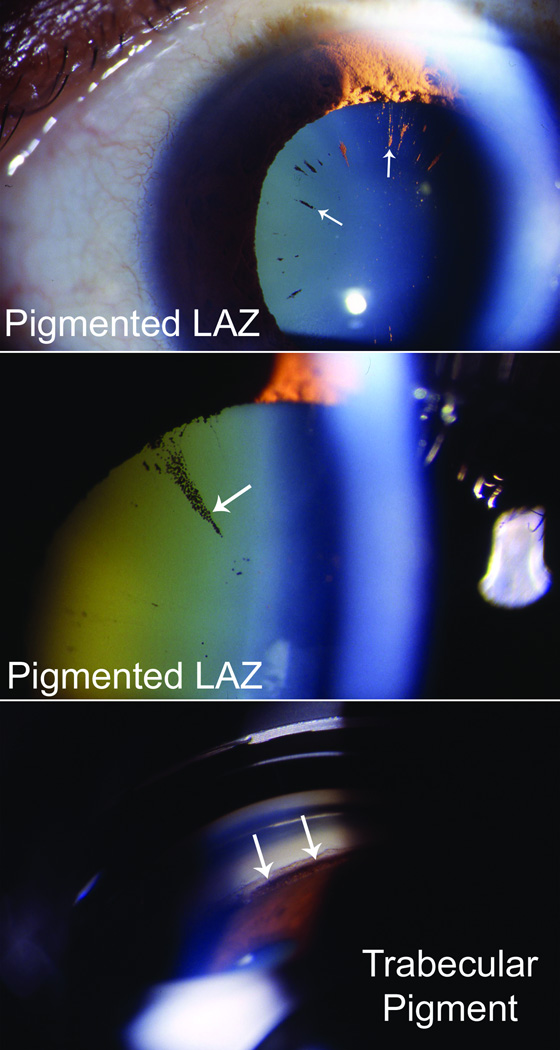
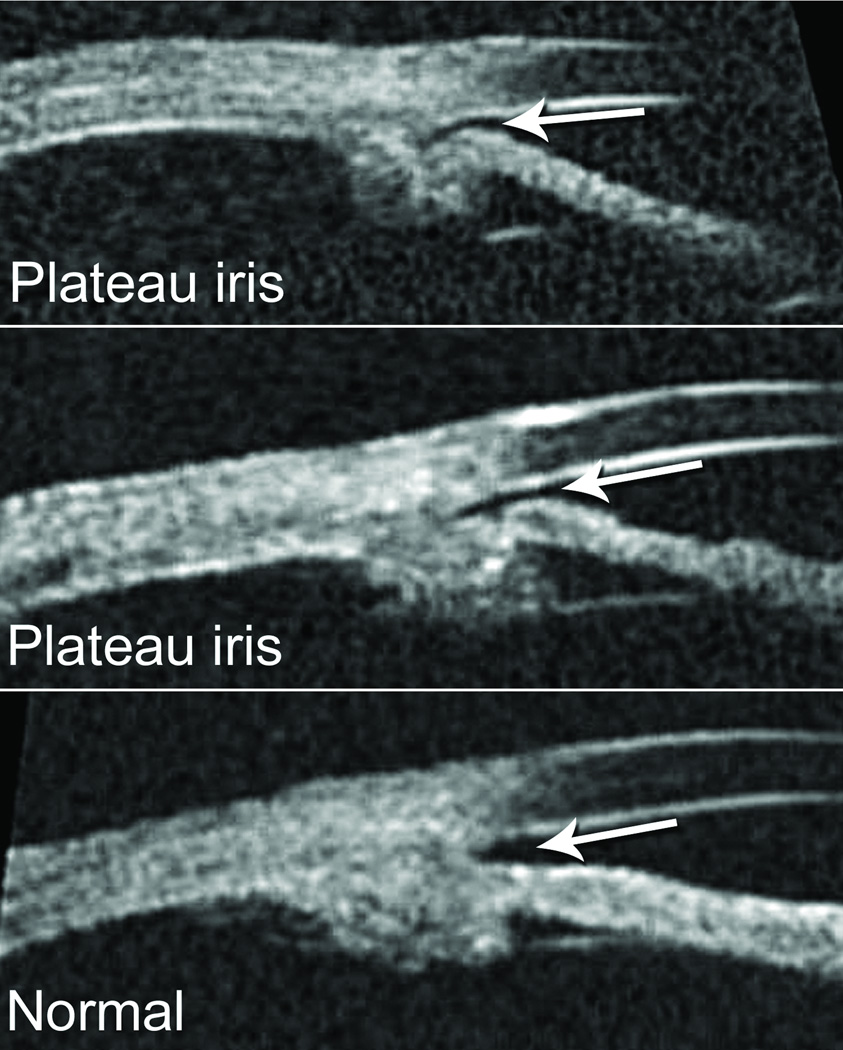
Long anterior zonules (LAZ) are characterized by the presence of crystalline lens zonules central to the normal insertion zone on the anterior capsule (Fig. 1),(1–12) sometimes resulting in a very reduced zonule-free zone (Fig. 2). Although LAZ may occur with late-onset retinal degeneration (L-ORD),(1;13) another variety occurs apart from L-ORD, most commonly in older, hyperopic women.(2;3;5–11) LAZ often become pigmented due to contact with the posterior iris, and other pigment dispersion signs are also common (Fig. 3) that can be confused with “classic” pigment dispersion syndrome.(3;4;8–11;14;15) Possibly, there is elevated risk for glaucoma in LAZ eyes, but definitive association is unknown. In addition to open-angle mechanisms, narrow-angle mechanisms might also increase the likelihood of glaucoma in LAZ eyes (Fig. 4).(16) Observations of hyperopia with LAZ are consistent with increased risk of angle-closure but no information currently exists about other ocular parameters. Therefore, we began study of ocular dimensions in LAZ eyes.
Figure 1.
Termination of normal anterior zonules along a well-demarcated zone (top) vs. irregular termination points of LAZ (bottom).
Figure 2.
Reduced zonule-free zone in subject with cataract and LAZ.
Figure 3.
LAZ-associated pigment dispersion resulting in densely pigmented long zonules (top, middle) and heavy pigmentation of the trabecular meshwork (bottom).
Figure 4.
Plateau iris configuration in subjects with the LAZ trait (top, middle) compared with a normal iris configuration (bottom).
Methods
Subjects were recruited from a single, urban, eye care facility in Chicago, Illinois, USA, and those selected for analysis presented as part of a larger investigation exploring the LAZ phenotype, co-morbidity associations, and potential heredity. The vast majority of the LAZ subjects were identified from a database developed over a 10–12 year period through the provision of routine eye care in the facility’s primary eye care service. Four of the LAZ inclusion subjects, with diagnoses including diabetic retinopathy, retinoschisis, macular degeneration, and central retinal vein occlusion, were initially identified and referred to the study from the Retina Service, but no other subjects were referred from any specialty service. LAZ participants responded to a mailed invitation, and only African-Americans (self-report) were studied due to institutional patient demographics. The criterion for LAZ was zonule fibers present ≥1.0 mm central to the normal zonule termination zone on the anterior lens surface (Fig. 1), and we excluded eyes with <5 LAZ to ensure definitive cases. Eyes with prior trauma or surgery were excluded, except for those having laser iridotomy for narrow angles.
Controls were frequency-matched to cases on race, gender, and age (5-year increments), and were derived from a database of more than 5,500 consecutive patients examined by five practitioners who screened for LAZ from 1999 to 2001 during provision of primary eye care. Potential controls were excluded if there was history of trauma, ocular surgery, or other significant eye disease. Possible inclusions were sent mailed invitations.
Testing included ocular/medical history, extraocular muscle testing, Randot™ stereopsis, Ishihara’s Test for Color Deficiency, pupil testing, auto-refraction/keratometry, subjective refraction, Goldmann tonometry, four-mirror gonioscopy, dilated fundus exam, stereo fundus photography, corneal pachymetry, A-scan ultrasonography, optic nerve confocal scanning laser tomography (HRT II), and Humphrey Field Analyzer II-Series central threshold testing.
Ocular parameters compared included non-dilated subjective refraction (SR), central corneal curvature (CCC), central corneal thickness (CCT), and axial length (AL). CCC was measured with the Humphrey Instruments Automatic Refractor/Keratometer, Model 599. CCT and AL were measured with the Palmscan™ AP2000 (MicroMedical Devices, Inc., Calabasas, CA). Spherical-equivalent values were used for SR comparisons, and the mean of the horizontal and vertical meridians were used for CCC. The mean of four consecutive automated readings were used for CCT, and the mean of six consecutive automated readings were used for AL. For CCC and AL measures, which could vary with probe position, the eye tested first was selected via randomization, and to assess repeatability, duplicate measures were obtained on a large number of consecutive subjects. Repeat measures were taken immediately after the first set of measures in the same right/left eye sequence. A single tester (DKR) took all CCC and AL measures.
To prevent diminution of subjects, imputations were carried out for missing data values by using the mean values of existing data for each subject group. There were only two instances in which either eye of a case/control group had more than two missing values. This included 9–10 missing CCC values for both eyes of LAZ subjects and four missing AL values for LAZ subject right eyes.
To examine variables, distributions were checked, and simple group comparisons were made using the Student’s t test. Right and left eyes were evaluated separately due to lack of independence and to provide experimental check for consistency. Multiple logistic regression was used to check for residual age confounding and to explore variable combinations for predictive relationships with LAZ presence. Explanatory variables were checked for correlation and interaction, and the likelihood ratio test was used to help select final models. Analyses were carried out using SAS® Statistical Program, Version 9.2 for Microsoft Windows® (Cary, NC). Institutional Review Board approval was obtained for this research.
Results
There were 191 LAZ subjects in the existing dataset at time of analysis, and 72 (mean age ± SD=70.5 ± 9.6 years; range=51–92 years) participated in the larger LAZ study. Eleven (mean age=81.0 ± 6.7 years, 69–88 years, 9 females) of these 72 were excluded from the current analysis, four because they had cataract surgery, and seven due to no matched control. Therefore, 61 proband LAZ subjects (55 females) were included, with 130 (112 females) from the database not used. Gender was similar (90.2% vs. 86.2% female; P=0.44), but the inclusions were younger than those not included (69.0 ± 8.9 years, 51–91 years vs. 76.7 ± 10.8 years, 37–101 years; P<0.0001). Mean age of the 61 LAZ inclusions was similar to controls (68.4 ± 8.3 years, 51–93 years; P=0.73). Mean age of the female LAZ cases was 68.4 ± 9.1 years (51–91 years) and 74.0 ± 5.4 years (68–81 years) for the males. Among the 61 LAZ cases, 7 subjects (5 females, mean age=71.2 years, 60–76 years; 2 males, 76 and 79 years) had previous laser iridotomy (all bilateral but one). Four of the LAZ subjects had definitive glaucoma, defined by characteristic optic nerve cupping and consistent visual field loss. All four were taking chronic topical intraocular pressure lowering medication, and they also had a history of intraocular pressure > 22 mm Hg in the presence of open-angles. Two of the subjects with chronic open-angle glaucoma were also subjects who had undergone laser iridotomy procedures for concern of angle narrowing at some point in time.
Significant differences existed between the LAZ and control eyes relative to SR and AL, but not CCC and CCT (Table 1). LAZ right eyes had a mean SR = +1.75 D ± 1.82 D and on average they were 1.58 D (95% CI=0.83 to 2.31 D, P<0.0001) more hyperopic than control right eyes. On average, LAZ right eyes were also 0.69 mm (95% CI=0.34 to 1.04 mm, P<0.001) shorter than control right eyes. Left eye comparisons were similar. Results were stable with the exclusion of subjects with missing data, subjects who had laser iridotomy, and males. “Difference vs. mean plots” for the CCT and AL repeat measures showed similar means and random distributions for the first vs. second trial differences.(17) Mean difference ± 95% limits of agreement for CCT measurements were 4.1 ± 20.5 u for right eyes and −2.6 µ ± 20.4 µ for left eyes. For AL they were −0.02 mm ± 0.28 mm for right eyes and 0.00 mm ± 0.47 mm for left eyes. None of the mean differences were statistically significant (P>0.05).
TABLE 1.
OCULAR PARAMETERS OF LAZ CASES AND MATCHED CONTROLS
|
LAZa Cases N=61 Mean±SD (range) |
Controls N=61 Mean±SD (range) |
|||
|---|---|---|---|---|
| Variable | Right Eye | Left Eye | Right Eye | Left Eye |
|
Corneal Thickness (µ) |
535±31 (475 to 615) |
526±29 (470 to 595) |
535±36 (446 to 617) |
529±36 (434 to 618) |
|
Corneal Curvature (D) |
43.35±1.36 (39.50 to 46.00) |
43.37±1.41 (39.50 to 46.00) |
43.50±1.31 (41.13 to 46.63) |
43.58±1.41 (40.75 to 46.50) |
| Axial Length (mm) |
**23.10±0.92 (21.36 to 25.29) |
**23.10±0.90 (20.93 to 25.30) |
23.79±1.01 (21.82 to 28.05) |
23.78±1.00 (22.06 to 28.12) |
|
Subjective Refraction(D) |
**+1.75±1.82 (−2.50 to ±7.25) |
**+1.58±1.74 (−2.38 to ±7.38) |
0.17±2.29 (−8.75 to ±3.25) |
−0.02± 2.24 (−8.38 to ±3.25) |
Abbreviations: D, diopters; mm, millimeters; µ, microns; SD, standard deviation
Significantly different from control eyes, P<0.001
Logistic regression confirmed good age-matching since results remained stable with simultaneous control for age. It also showed that other variables, and their combinations, were not superior to SR alone or AL alone as prediction variables for the case vs. control status (Table 2). Comparing regression equations with SR vs. AL as sole prediction variables for the case/control status, i.e., “LAZ (Y) = Intercept + SR (X1)” vs. “LAZ (Y) = Intercept + AL (X1)”, neither logistic model was superior to the other (P>0.05). In addition to SR and AL as sole predictor variables of the case/control status, analysis also showed that CCC and AL together in a regression equation could predict subject type. Here, for right and left eyes, while controlling for AL, the odds of being a LAZ case were higher (OR=1.5; 95% CI=1.1 to 2.1; P=0.017) with a flatter CCC.
TABLE 2.
LOGISTIC REGRESSION MODELS SHOWING RELATIONSHIP BETWEEN LAZ AND SIGNIFICANT PREDICTOR VARIABLES (61 CASES, 61 CONTROLS; 50–94 YEARS OLD)
| LAZ (Y) = Intercept + refractive error (X1) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Coefficient (β) |
SE | Wald ×2 | P-value | OR | 95% CI | |
|
Right Eye |
Intercept Refractive Error (D) |
−0.46 0.45 |
0.24 0.13 |
- 12.54 |
- 0.0004 |
- 1.57 |
- 1.22 to 2.01 |
|
Left Eye |
Intercept Refractive Error (D) |
−0.41 0.47 |
0.23 0.13 |
- 13.74 |
- 0.0002 |
- 1.60 |
- 1.25 to 2.06 |
| LAZ (Y) = Intercept + axial length (X1) | |||||||
| Variable |
Coefficient (β) |
SE | Wald ×2 | P-value | OR | 95% CI | |
|
Right Eye |
Intercept Axial Length (mm) |
−18.40 0.79 |
5.28 0.23 |
- 12.15 |
- 0.0005 |
- 2.19 |
- 1.41 to 3.41 |
|
Left Eye |
Intercept Axial Length (mm) |
−18.95 0.81 |
5.44 0.23 |
- 12.13 |
- 0.0005 |
- 2.25 |
- 1.42 to 3.54 |
| LAZ (Y) = Intercept + corneal curvature (X1) + axial length (X2) | |||||||
| Variable |
Coefficient (β) |
SE | Wald ×2 | P-value | OR | 95% CI | |
|
Right Eye |
Intercept Corneal Curvature (D) Axial Length (mm) |
−43.73 0.42 1.10 |
12.51 0.17 0.28 |
- 5.67 14.79 |
- 0.017 0.0001 |
- 1.52 2.99 |
- 1.08 to 2.14 1.71 to 5.23 |
|
Left Eye |
Intercept Corneal Curvature (D) Axial Length (mm) |
−47.74 0.46 1.19 |
12.69 0.17 0.30 |
- 7.22 15.94 |
- 0.007 <0.0001 |
- 1.58 3.29 |
- 1.13 to 2.21 1.83 to 5.89 |
Abbreviations: CI, confidence interval; D, diopters; mm, millimeters; OR, odds ratio; SE, standard error
Discussion
In addition to a relationship with hyperopia,(7–9) this study also finds a LAZ association with shorter axial length. The finding that LAZ eyes tend to be shorter is not surprising, but requires formal study nonetheless. Significance of hyperopia and shorter axial length is related to known association with angle-closure glaucoma,(18–21) as are older age and female gender, (22) which have consistently been found with LAZ in our clinic population.(7–9) Thus, based on observations to date, at least certain characteristics of African-American LAZ subjects, i.e., older age, female gender, hyperopia, and shorter axial lengths, are similar to classic descriptions of people who develop primary angle-closure glaucoma.(23)
Although steeper anterior surface CCC has been reported with angle-closure glaucoma in some subject groups,(21;24) our LAZ subjects did not exhibit this tendency. Alternatively, while controlling for AL, we found CCC was significantly more likely to be flatter among LAZ cases than controls, an observation that may be consistent with higher hyperopic refractive error. The contribution of other ocular components will be needed to fully understand this. In our current study, the ability to accurately measure other ocular parameters, such as anterior chamber depth and lens thickness, was constrained by our measurement method.
Angle-closure glaucoma is a complex disease with clinical variation from one population to another, and its incidence varies among ethnic groups.(25–27) Acute angle-closure is less common in blacks, who may tend to develop chronic angle-closure when it does occur.(27;28) We did not classify our subjects in terms of acute or chronic angle-closure among those who had it because the histories were not always clear. Although it is a goal to determine the level of open-angle and narrow-angle glaucoma risk among LAZ eyes, this information cannot be inferred from our type of data.
In addition to shorter axial lengths, angle-closure risk may be further increased if plateau iris configuration is truly more likely to occur in LAZ eyes.(16) Plateau iris, which can cause angle-closure stemming from abnormally positioned ciliary processes,(29–32) might be likely in LAZ eyes if certain structural changes inherent with LAZ create anomalous traction on the ciliary processes, causing their forward rotation with change in peripheral iris configuration.
The inclusion of only African-Americans in this study reflected study site demographics, and it is not the intent to suggest that LAZ or related features are limited to this group. This is because study at the same facility has suggested similar LAZ prevalence among other groups as well, i.e., about 2.0%.(8)
This study further adds to the body of literature that a basic clinical picture is emerging of a common patient phenotype characterized by older age, female gender, hyperopia, shorter axial length, intraocular pigment dispersion, and LAZ. In contrast to the younger age, male gender, myopia, and longer axial length that may characterize classic pigment dispersion syndrome,(33) LAZ subjects usually have characteristics opposite these and could be more likely to develop angle-closure glaucoma, with possible contribution from plateau iris.
Conclusions
LAZ eyes in this African-American dataset tended to be more hyperopic and have axial lengths shorter than control eyes, characteristics that are consistent with elevated risk for angle-closure glaucoma.
Acknowledgments
Grant Support:
National Eye Institute Grant K23 EY0181883 (DKR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ayyagari R, Mandal MN, Karoukis AJ, Chen L, McLaren NC, Lichter M, et al. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci. 2005;46(9):3363–3371. doi: 10.1167/iovs.05-0159. [DOI] [PubMed] [Google Scholar]
- 2.Bellows JG. Pigmented lines in retroiridal region of anterior capsule of lens. Arch Ophthalmol. 1944;32:483–484. [Google Scholar]
- 3.Larsen FE, Hvidberg J. Vogt's retro-iridian pigment lines. Light microscopical and ultrastructural study of a case. Acta Ophthalmol (Copenh) 1976;54(5):641–653. doi: 10.1111/j.1755-3768.1976.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 4.Moroi SE, Lark KK, Sieving PA, Nouri-Mahdavi K, Schlotzer-Schrehardt U, Katz GJ, et al. Long anterior zonules and pigment dispersion. Am J Ophthalmol. 2003;136(6):1176–1178. doi: 10.1016/s0002-9394(03)00657-3. [DOI] [PubMed] [Google Scholar]
- 5.Ohrt V. Diabetic Iridopathy: Clinical Studies of the Pigment Layer of the Iris, Pupillary Function and Rubeosis Iridis in Diabetic Patients. Denmark: Universitetsforlaget I Aarhus; 1967. [PubMed] [Google Scholar]
- 6.Rieger G. Radial retroiridal linear pigmentation. Br J Ophthalmol. 1981;65(11):760–761. doi: 10.1136/bjo.65.11.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts DK, Winters JE, Castells DD, Clark CA, Teitelbaum BA. Pigmented striae of the anterior lens capsule and age-associated pigment dispersion of variable degree in a group of older African-Americans: an age, race, and gender matched study. Int Ophthalmol. 2001;24(6):313–322. doi: 10.1023/b:inte.0000006762.32723.17. [DOI] [PubMed] [Google Scholar]
- 8.Roberts DK, Lo PS, Winters JE, Castells DD, Alexander CC, Teitelbaum BA. Prevalence of pigmented lens striae in a black population: a potential indicator of agerelated pigment dispersal in the anterior segment. Optom Vis Sci. 2002;79(11):681–687. doi: 10.1097/00006324-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DK, Winters JE, Castells DD, Teitelbaum BA, Alexander CC. A cross-sectional study of Krukenberg spindles and pigmented lens striae in a predominately black population: two highly associated clinical signs of anterior segment pigment dispersal. J Glaucoma. 2005;14(1):57–63. doi: 10.1097/01.ijg.0000146368.21001.88. [DOI] [PubMed] [Google Scholar]
- 10.Stankovic M, Stankovic I. Les lignes pigmentées épicapsulaires du cristallin (Vogt) et le glaucome. Bulletins et memoires de la societe francaise d'ophthalmologie. 1962;75:586–595. [Google Scholar]
- 11.Sturrock GD, Tripathi RC. Pigmented lens striae. Br J Ophthalmol. 1976;60(4):287–293. doi: 10.1136/bjo.60.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogt A. Lehrbuch und Atlas der Spaltlampenmikroskopie. 2nd ed. Berlin: Springer: 1931. [Google Scholar]
- 13.Subrayan V, Morris B, Armbrecht AM, Wright AF, Dhillon B. Long anterior lens zonules in late-onset retinal degeneration (L-ORD) Am J Ophthalmol. 2005;140(6):1127–1129. doi: 10.1016/j.ajo.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DG. Pigmentary dispersion and glaucoma. A new theory. Arch Ophthalmol. 1979;97(9):1667–1672. doi: 10.1001/archopht.1979.01020020235011. [DOI] [PubMed] [Google Scholar]
- 15.Karickhoff JR. Pigmentary dispersion syndrome and pigmentary glaucoma: a new mechanism concept, a new treatment, and a new technique. Ophthalmic Surg. 1992;23(4):269–277. [PubMed] [Google Scholar]
- 16.Roberts DK, Ayyagari R, Moroi SE. Possible association between long anterior lens zonules and plateau iris configuration. J Glaucoma. 2008;17(5):393–396. doi: 10.1097/IJG.0b013e31815c3b04. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 18.Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54(3):161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugar HS. The mechanical factors in the etiology of acute glaucoma. Am J Ophthalmol. 1941;24(8):851–872. [Google Scholar]
- 20.Lowe RF. Primary angle closure glaucoma: a review of ocular biometry. Aust J Ophthalmol. 1977;5:9–17. [Google Scholar]
- 21.Tomlinson A, Leighton DA. Ocular dimensions in the heredity of angle-closure glaucoma. Br J Ophthalmol. 1973;57(7):475–486. doi: 10.1136/bjo.57.7.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luntz MH. Primary angle-closure glaucoma in urbanized South African caucasoid and negroid communities. Br J Ophthalmol. 1973;57(7):445–456. doi: 10.1136/bjo.57.7.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage JA. Primary angle-closure glaucoma. In: Zimmerman TJ, Kooner KS, editors. Clinical Pathways in Glaucoma. New York: Thieme Medical Publishers, Inc.; 2001. pp. 81–106. [Google Scholar]
- 24.Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin Experiment Ophthalmol. 2000;28(4):253–258. doi: 10.1046/j.1442-9071.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Alsbirk PH. Angle-closure glaucoma surveys in Greenland Eskimos. A preliminary report. Can J Ophthalmol. 1973;8(2):260–264. [PubMed] [Google Scholar]
- 26.Lowe RF. Comparative incidence of angle-closure glaucoma among different national groups in Victoria, Australia. Br J Ophthalmol. 1963;47:721–727. doi: 10.1136/bjo.47.12.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alper MG, Laubach JL. Primary angle-closure glaucoma in the American Negro. Arch Ophthalmol. 1968;79(6):663–668. doi: 10.1001/archopht.1968.03850040665003. [DOI] [PubMed] [Google Scholar]
- 28.Clemmesen V, Luntz MH. Lens thickness and anglo-closure glaucoma. A comparative oculometric study in South African Negroes and Danes. Acta Ophthalmol (Copenh) 1976;54(2 p):193–197. [PubMed] [Google Scholar]
- 29.Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113(4):390–395. doi: 10.1016/s0002-9394(14)76160-4. [DOI] [PubMed] [Google Scholar]
- 30.Ritch R. Plateau Iris Is Caused by Abnormally Positioned Ciliary Processes. J Glaucoma. 1992;1:23–26. [Google Scholar]
- 31.Tornquist R. Angle-closure glaucoma in an eye with a plateau type of iris. Acta Ophthalmol (Copenh) 1958;36(3):419–423. doi: 10.1111/j.1755-3768.1958.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 32.Wand M, Grant WM, Simmons RJ, Hutchinson BT. Plateau iris syndrome. Trans Am Acad Ophthalmol Otolaryngol. 1977;83(1):122–130. [PubMed] [Google Scholar]
- 33.Farrar SM, Shields MB. Current concepts in pigmentary glaucoma. Surv Ophthalmol. 1993;37(4):233–252. doi: 10.1016/0039-6257(93)90008-u. [DOI] [PubMed] [Google Scholar]