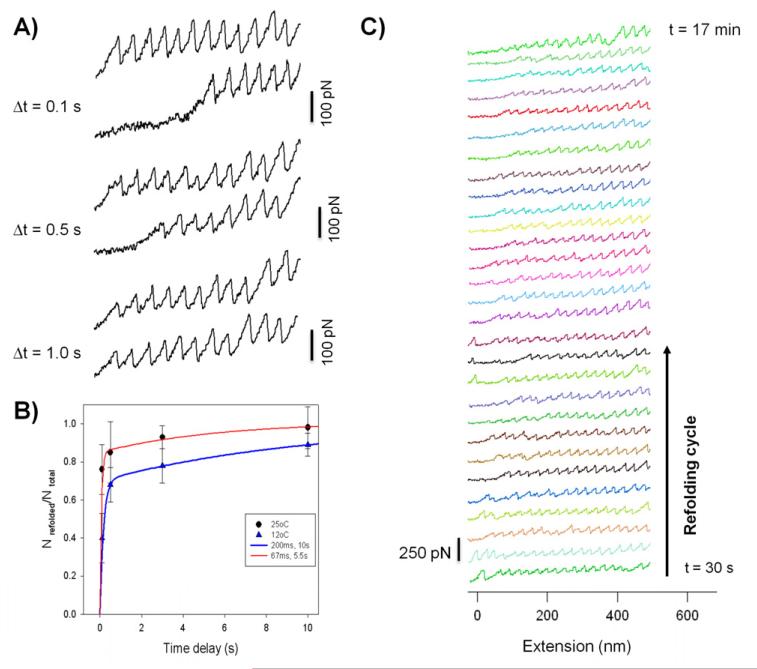
Figure 4. Unfolding and refolding kinetics of single titin-like proteins.
A) Refolding of projectin domains depending on relaxation time (100ms, 1s, 10s). The refolding probability increases with increasing the time interval between stretching pulses. After 10s relaxation almost all domains refolded into their native state. B) Fraction of refolded domains as a function of the time delay between stretching pulses. The solid lines are a two-exponential fit of the data to the function Nrefolded/Ntotal =A1 (1 − e−t·β 1) + A2 (1 − e−Δt·β 2). Considering the heterogeneous nature of the Ig-like domains, we attribute the biphasic folding kinetics to heterogeneity in folding kinetics among the different domains of projectin. C) A series of force-extension and relaxation cycles collected from a single projectin molecule. The delay time between each refolding cycle was about 20 seconds. In this example the protein was unfolded and refolded a total of 52 times. Reproduced with permission from (100).