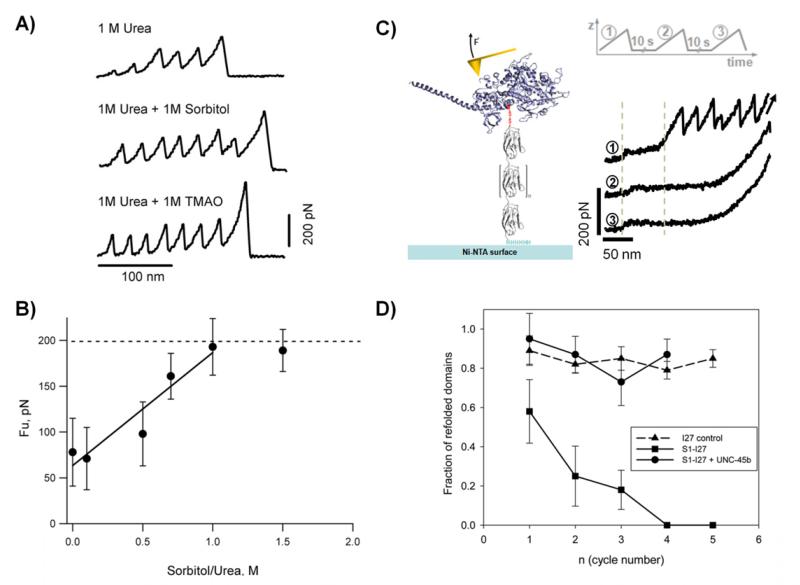
Figure 6. Probing the effects of osmolytes and molecular chaperones on protein folding and stability.
A) Typical force-extension traces for a polyPKDd1-I27 protein obtained in different conditions: 1 M urea, 1 M urea + 1 M sorbitol, and 1 M urea + 1 M TMAO. B) Plot of the unfolding forces for PKD domains as a function of the sorbitol/urea ratio. The unfolding forces of PKDd1 steadily increases from 78 at 0 M, 71 at 0.1 M, 98 at 0.5 M, 161 at 0.7 M, to 193 at 1 M. The line represents a linear fit to the experimental data obtained below 1.0 sorbitol/urea ratio. C) Left) Diagram of a titin-derivatized myosin motor domain. Myosin (blue) was derivatized with a mechanical reporter (eight repeats of the titin I27 domain) carrying an N-terminal cysteine residue and a C-terminal His6 tag. C) Right) Single derivatized myosins were subjected to repeated cycles of mechanical stretching, separated by a waiting time of 10 seconds at zero force. The characteristic I27 sawtooth pattern is seen in the initial unfolding trace (1). It is not present in the following traces (2 and 3), indicating that the unfolded myosin interferes with refolding of the I27 domains. The low-force plateau (around 30 pN) represents the unfolding of the myosin rod domain showing that it refolds independently from the motor domain. D) Plot of the refolding probability of titin I27 domains as a function of the stretching/relaxation cycles. Single S1-I27 proteins were subjected to repeated cycles of unfolding in the absence of chaperone (squares) or in the presence of 1 μM UNC-45 (circles). As a control the same experiment was performed using the I27 polyprotein (triangles). Reproduced with permission from (35, 36).