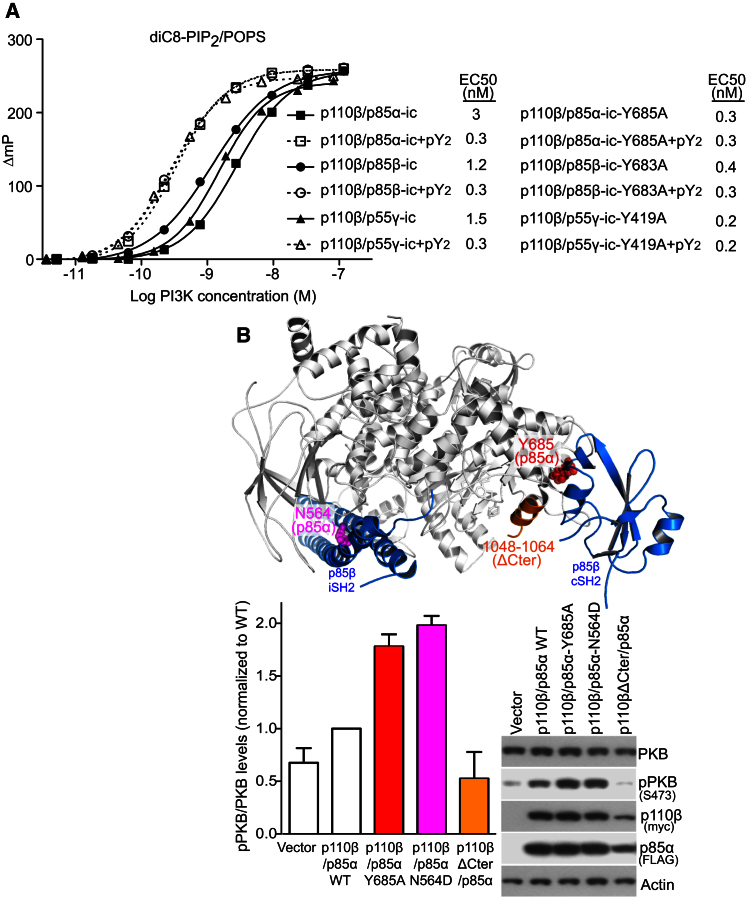
Figure 4.
cSH2 Is Important for p110β Inhibition In Vitro and in Cells
(A) Kinase activity of human p110β and icSH2 wild-type constructs from three human regulatory subunits (p85α, p85β, and p55γ) in the absence and presence of 10 μM PDGFR pY2 (y axis as in Figure 1B). EC50s for wild-type and icSH2 tyrosine mutants, in the absence and presence of 10 μM PDGF pY2, are shown.
(B) Western blots of HEK cells transiently expressing wild-type and mutant human p110β/p85α. The membrane was probed with antibodies against PKB, pPKB (pSer473), myc (p110β), FLAG (p85α), and actin. Bar graphs show mean ± SEM (n = 3) of PKB phosphorylation level normalized to wild-type p110β/p85α (WT). The position of the three mutations is mapped on the p110β/p85β structure.