Summary
The aim of this study was to develop a technically simple swine aneurysm-training model by inserting a silicone aneurysm circuit in the cervical vessels.
A silicone aneurysm circuit was created by designing multiple aneurysms in size and configuration on a silicone vessel. Five swine underwent surgical implantation of this circuit in the cervical vessels: one end in the common carotid artery and the other in the external jugular vein. Using this model, an aneurysm coiling procedure was simulated under fluoroscopic guidance, roadmapping and digital subtraction angiography.
Creating an aneurysm model for training purposes by this method was technically simple and enabled the formation of a wide variety of aneurysms in a single procedure. The quality of the model was uniform and the model was reproducible. Coiling training using this model resembled a realistic clinical situation.
The swine hybrid aneurysm-training model was advantageous from the standpoint of technical simplicity in the creation and variety of aneurysms it provided. The swine hybrid aneurysm model may be an additional option for aneurysm coiling training.
Key words: interventional training, aneurysm, animal model, swine
Introduction
Endovascular coiling of cerebral artery aneurysms has been increasingly performed since the publication of the landmark ISAT study 1. However, due to rapid advancements in technique and device, updating oneself with the quickly shifting environment is challenging. For instance, six new types of coil and one intracranial stent for aneurysm coiling were approved for clinical use in Japan in the past two years. Therefore, development of a realistic training model to familiarize oneself with new devices is necessary to perform safe procedures.
To date, a number of reports on aneurysm models have been published. These include in vitro silicone 2 and simulators 3 as well as in vivo animal models. Animal models with surgically created aneurysms have a wide range of diversity from animal species to the aneurysm configuration, depending on the aneurysm creation method. Reported models include venous pouch implantation sidewall and bifurcation models in murine 4 canine 5,6, swine 7,8, and rabbit 9,10 models. An endovascularly created elastase-induced rabbit bifurcation aneurysm model has been also reported 11.
For the purpose of understanding aneurysm physiology or testing new endovascular devices, aneurysm models should resemble the features of human cerebral artery aneurysms as closely as possible. On the other hand, for intervention training purposes, a simple yet realistic model is desired. In vitro models such as silicone or simulators are simple in preparation but lack the realistic environment of aneurysm coiling. In vivo animal models are ideal in resembling a realistic coiling situation. However, preparation of the model is time-consuming and the configuration of the aneurysm is often too simple compared to human cerebral aneurysms. Repetitive practice in a standardized condition is also difficult to achieve with in vivo models.
To overcome the drawbacks of the present in vitro and in vivo aneurysm models for training use, we have developed a novel hybrid swine aneurysm model. This model features a silicone aneurysm circuit implanted into swine cervical vessels. The concept of the model was to create a wide variety of aneurysms for intervention training purposes with reduced animal preparation effort, while maintaining a lively intervention-training environment. This article describes the development of the model and a preliminary training session using this model.
Materials and Methods
Aneurysm circuit model
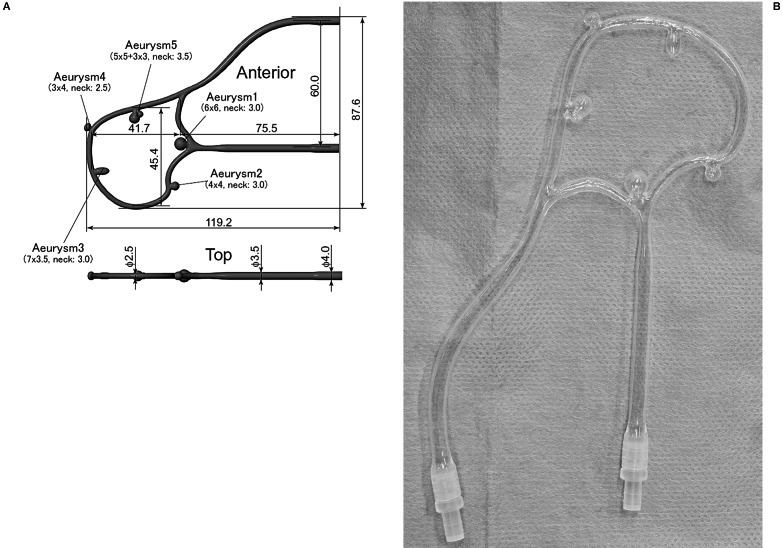
The aneurysm circuit model was made from silicone and was designed so as to simulate bifurcation and sidewall, simple and complex aneurysm coiling (Figure 1A). Hubs were attached on both ends of the circuit to connect an extending silicone vessel and a stop-cock (Figure 1B).
Figure 1.
A) Aneurysm circuit is designed to simulate bifurcation and side wall, simple and complex aneurysm coiling. Measurements in the figure are in mm. B) The aneurysm circuit is made from silicone. Hubs on both ends enable stop-cock and extending silicone vessel connection, which in turn are connected to catheters inserted in swine carotid vessels.
Animal preparation and model construction
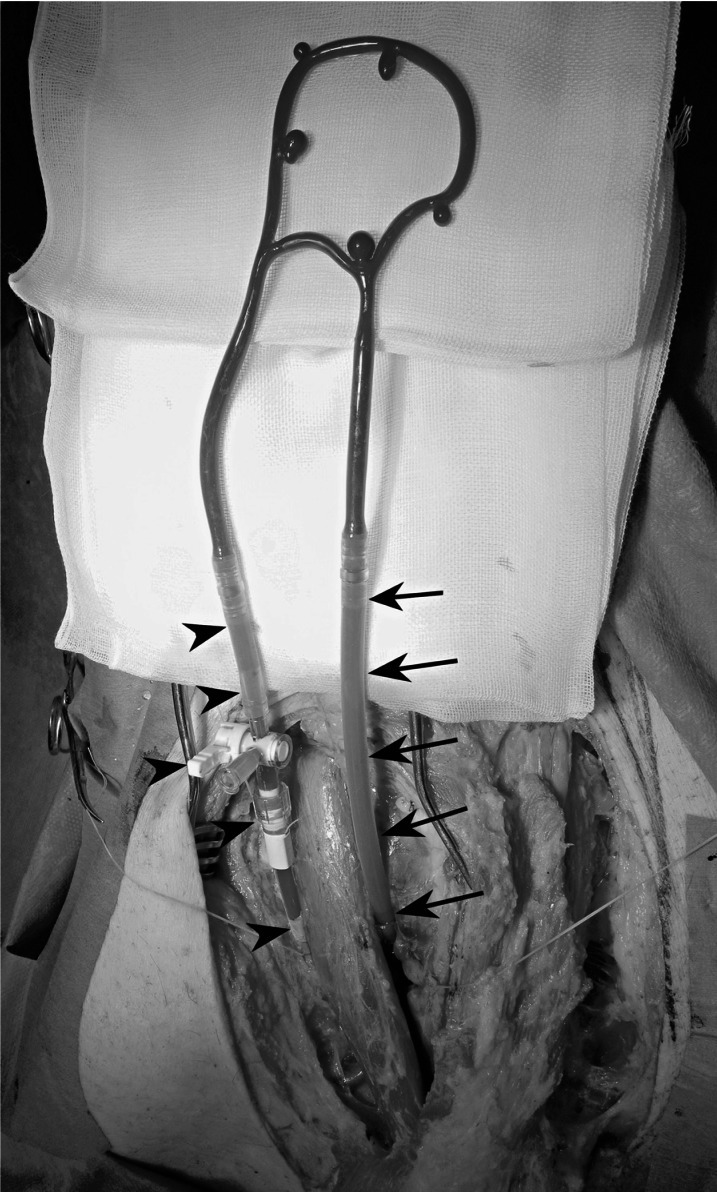
The study was conducted according to the institutional regulations and guidelines for animal experiments, under approval of the institutional animal experiment ethics committee. All procedures were performed in 35-40 kg swine (two Mexican hairless, and three domestic pigs) under general anesthesia. Following induction of anesthesia, right external jugular vein and common carotid artery were exposed through a midline or para-midline linear incision between the larynx and sternum. Then the ends of the silicone aneurysm circuit were inserted in the common carotid artery and ipsilateral external jugular vein. To insert the circuit, both vessels were clamped and punctured with 16 gauge catheter-over-needle assemblies. The catheters were advanced into the vessels and fixed with 2-0 silk sutures. Then both ends of the circuit were connected via an extending silicone tube in the arterial side, and a stop-cock in the venous side. The vessels were then declamped, and air in the circuit was suctioned out from the stop-cock in the venous side using a syringe (Figure 2). In the first case, the circuit was connected to the proximal and distal common carotid artery establishing an antegrade arterial blood flow through the circuit. Following aneurysm circuit insertion, the swine was systemically heparinized with an intravenous injection of 100 units/kg of heparin.
Figure 2.
The aneurysm circuit is inserted into the swine carotid vessels. The left side of the circuit is inserted into the right common carotid artery via an extending silicone tube and a catheter (arrows). The right side is inserted into the external jugular vein via a stop-cock (arrowheads).
Endovascular surgery
In the last experiment, a preliminary coiling procedure was performed by a senior interventional surgeon and a trainee. Arterial access with an 11 cm 5-Fr sheath (Terumo, Tokyo, Japan) was obtained by either femoral artery exposure or percutaneous medial saphenous artery approach. This was followed by step by step simulation of an endovascular technique: 5-Fr Envoy guiding catheter (Cordis, Miami, FL, USA) advancement, Echelon 10 microcatheter (Covidien ev3, Irvine, CA, USA) catheterization of aneurysms over a Silverspeed 10 microguidewire (Covidien ev3), and Axium coil (Covidien ev3) placement. Fluoroscopic guidance and DSA were obtained using Axiom Artis U (Siemens Medical Solutions, Erlangen, Germany) as required. Surgical time, fluoroscopic time, radiation dose, and radiation exposure of the surgeon and animal were documented.
Results
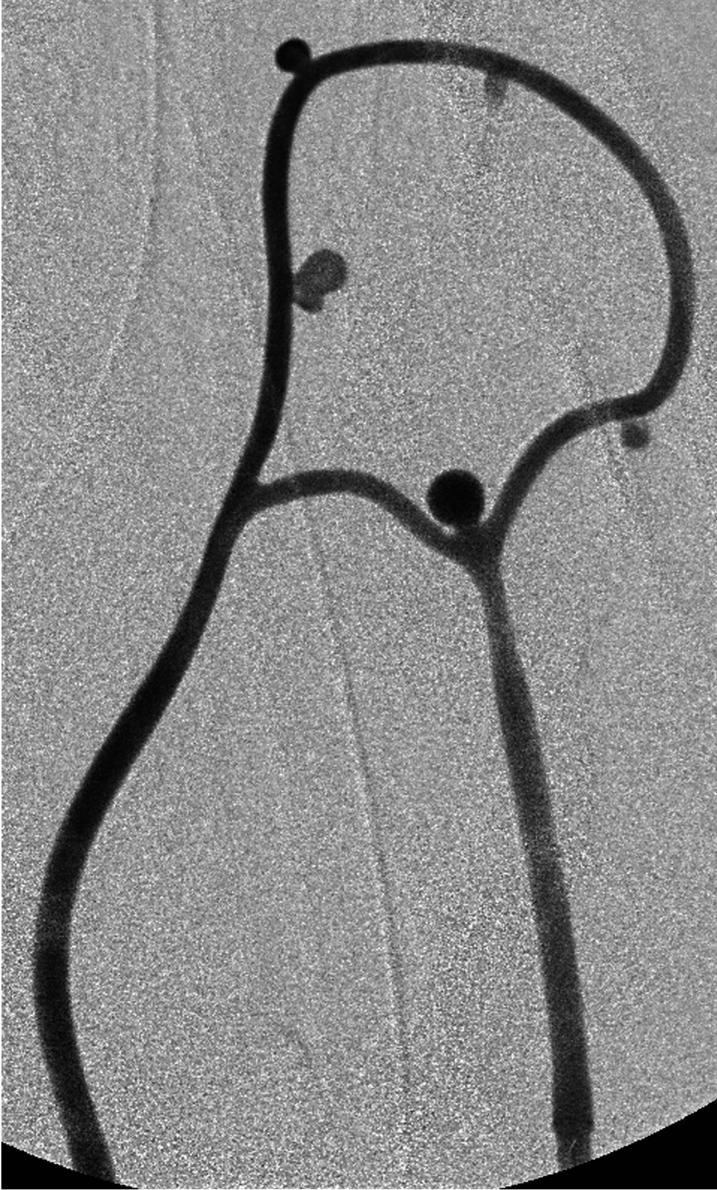
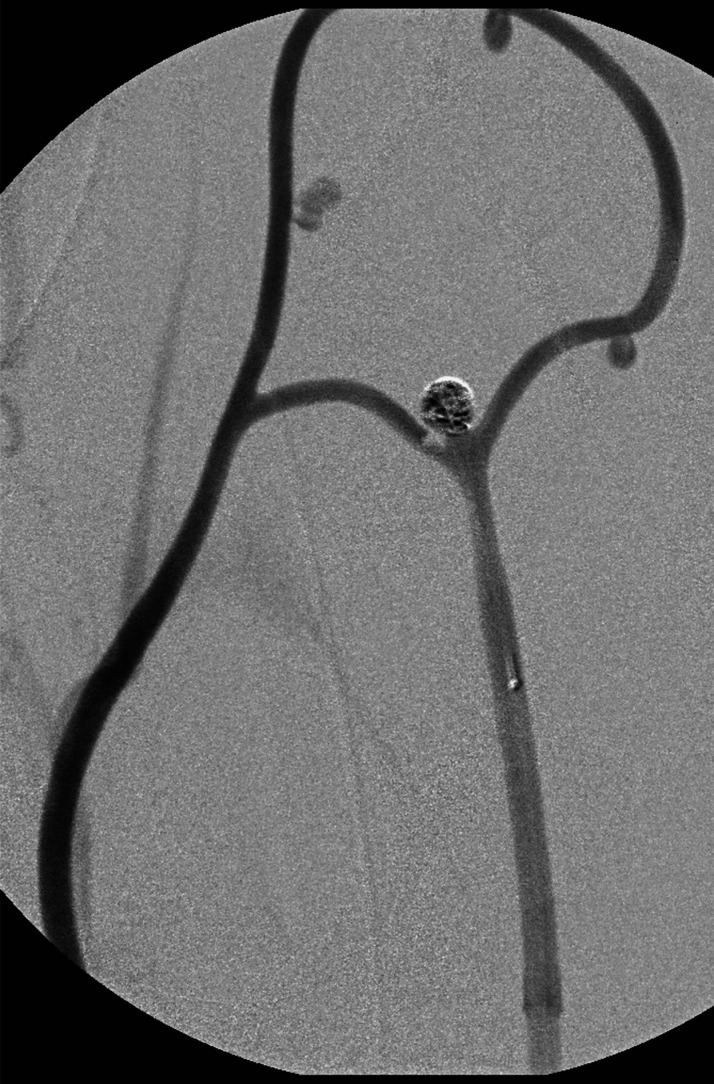
In five animals, the aneurysm-training model was successfully created without complications. Mean time to create the model was three hours, although experience and refinements in technique led to less surgical effort, resulting in two hours at the last experiment. In the first experiment, in which the aneurysm circuit was connected between the proximal and distal common carotid artery, thrombus occluded the circuit despite systemic heparinization. After the first experiment, the circuit was inserted between the common carotid artery and ipsilateral external jugular vein. This improvement secured continuous blood flow through the circuit, eliminating thrombus occlusion. DSA confirmed successful creation and patency of the aneurysm model (Figure 3). Using this model, a preliminary aneurysm coiling training was successfully performed by a senior interventional surgeon and a trainee (Figure 4). A thrombus is seen in the right corner of the coiled aneurysm. Training was conducted to simulate the real aneurysm coiling: guiding catheter placement, microcatheter advancement over a microguidewire, and coil insertion. Surgery time, fluoroscopic time, radiation dose, and radiation exposure of the surgeon and animal are shown in Table 1. The trainee required longer surgery time and a higher radiation dose, which may indicate the technical difficulty involved in the procedure and the validity of the model in developing interventional skills.
Table 1.
Surgery and radiation parameters in a senior surgeon and trainee
| Surgery time |
Fluoroscopic time |
Radiation dose |
Surgeon exposure |
Animal exposure |
|
|---|---|---|---|---|---|
| Trainee | 59'32” | 24'00” | 2500 μGy | 4 μSv | 118 μ956;Sv |
| Senior | 24'30” | 13'03” | 714.2 μGy | 1 μSv | 71 μSv |
Figure 3.
Digital subtraction angiogram demonstrates successful creation of five aneurysms for coiling training.
Figure 4.
A preliminary coiling training was performed by a interventional trainee from guiding catheter placement, microcatheter advancement to bifurcation aneurysm coil insertion. Note a thrombus at the right corner of the coiled aneurysm.
Discussion
Animal aneurysm models have mainly been developed for the purpose of evaluating endovascular devices and studying aneurysm physiology while few have been designated for interventional training 12-14. However, to provide maximal patient service, training young interventionalists is as important as the evolution of the scientific aspect of aneurysms. Familiarity with interventional skills and devices is desired before human application of this technique. Training using an animal aneurysm model is ideal to achieve this.
The two most common aneurysm models in the literature that have been proved valuable for the assessment of aneurysm devices are the canine bifurcation and the rabbit elastase-induced models 15. The canine bifurcation model has a tendency to recur after coil embolization, which makes it ideal for long-term aneurysm device assessment. Rabbits are easy to handle and the elastase-induced aneurysm requires less surgical effort since the endovascular technique is used for its creation. However, for the purpose of training, these models may be suboptimal. For the canine bifurcation model, the surgical effort to create the aneurysm may be a possible limitation. From personal experience, it usually takes four to five hours to create an aneurysm 16. The rabbit elastase-induced aneurysms may be uncontrolled in size and require approximately a one-month incubation period for aneurysm maturation. In addition, a surgically created aneurysm is often single and lacks the complex configuration often encountered in the real clinical setting. These factors may preclude diverse coiling training experience.
Silicone models and simulators may be alternatives to animal models for training purposes 2,3. These have two major advantages compared to animal models. First, they avoid ethical problems involved in animal experiments. Second, they are cost effective. Repetitive training is possible without animal lives, developing a basic understanding of the multiple steps involved in the interventional procedure. On the other hand, they may not provide a realistic environment, the sequence of the surgical procedure, and subtleties involved in the manipulation of interventional devices. As a consequence, their benefit may be limited to beginners who are new to this field 3.
Our hybrid swine aneurysm model implants a silicone aneurysm circuit into swine cervical vessels and was aimed solely for interventional training purposes. We are aware that there are criticisms regarding the use of an animal, the need for an animal surgeon, a lengthy surgical time, and the use of a silicone circuit that has altered friction coefficients compared to biological aneurysms. Accepting the caveats described above, we would still propose the advantages of our new model for three reasons. First, use of an animal model confers seriousness and reality to the training environment that will enhance the motivation of the trainees. These environmental elements are difficult to reproduce with simulators and silicone models. Since objective evaluation of these elements is difficult, they are poorly addressed in previous publications. However, we believe that successful training relies a great deal on the motivation of the trainees. Therefore, as although an animal based training seems futile, there is a certain role of animal models in interventional training.
Second, the procedure itself closely resembles the entire procedure of aneurysm coiling. From guiding catheter insertion to coiling, process and technique, such as fluoroscopic guidance, backflushing of catheters, roadmapping, selective catheterization of target vessels, and digital subtraction angiography are identical to the real clinical setting. This will help trainees understand an overview of the surgical procedure.
Third, the silicone component occupies only a small proportion in the entire model as shown in Figure 2. As a result, the silicone component did not cause friction that would have negatively impacted on the subtlety of the procedure.
An ideal animal aneurysm model for training purposes may be expected to have the following properties: 1) simple and easy to create within a short amount of time, 2) wide varieties of aneurysms enabling application of various coiling techniques, 3) uniformity to ensure training quality, 4) simulation of a realistic environment of aneurysm coiling in a surgical angiographic suit, 5) sustainable low cost.
Our training model was developed to fulfil the requirements listed above. Surgery time was three hours on average, which was reduced to two hours with accumulating experience. The aneurysm circuit was designed to resemble multiple types of aneurysms encountered in clinical practice. By using the aneurysm circuit, a uniform quality of the model was obtained. The circuit was durable and the same circuit was reused throughout the experiment, reducing the cost of constructing this model. Thus, we consider that this model met the requirements of the animal aneurysm training model listed above. Obviously, some improvements are needed in our silicone aneurysm circuit. First, our model lacks the tortuosity such as the carotid siphon encountered in human cerebral artery catheterization. For advanced training, a more complex circuit with tortuous curves proximal to the aneurysms is necessary. Second, despite systemic heparinization, thrombus formation was seen in our circuit. In the first experiment, in which the circuit was implanted in between the proximal and distal common carotid artery, a thrombus occluded the circuit. Likewise, as seen in Figure 4, the formation of small thrombi was frequently seen in the circuit. This was presumably due to the high resistance of the aneurysm circuit resulting in blood flow stasis. We believe that a large bore connecting system at both ends of the circuit will prevent the thrombosis. Additionally, aneurysm size and configuration could be redesigned to increase the technical challenge.
Our preliminary training system has some limitations. Efficacy of the model in developing interventional skills has yet to be validated. In the last experiment, a comparison between a senior surgeon and a trainee demonstrated more time and a higher radiation dose were required in the trainee. This may indicate that coiling simulation using this model could improve interventional skills of trainees. However, any definitive conclusion cannot be derived from this preliminary result. Assessment of the efficacy of our model with interventional trainees is currently ongoing. Second, there is the ethical concern regarding the use of animals. Third, because of animal use, the need for a specialized facility, and the short-term nature of this model, the cost-effectiveness is not high. Reasoning underlying animal use has already been discussed above in detail. However, ethical considerations for animal use should be given serious respect, and we admit that the future direction should aim to develop a better in vitro training system.
Conclusion
We have developed a novel hybrid aneurysm training model by implanting a silicone aneurysm circuit in swine cervical vessels. The model was created in a short amount of time with a technically simple surgical procedure. The swine hybrid aneurysm model may be an additional option for aneurysm coiling training.
Acknowledgments
This study was supported in full funding by Grant-in-Aid for Scientific Research (C) # 22591598, Grant-in-Aid for Scientific Research (KAKENHI), Japan Society for the Promotion of Science.
References
- 1.International subarachnoid aneurysm trial (ISAT) collaborative group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms, a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Fujitsuka M, Chaloupka JC, et al. Simulation of endovascular neurointervention using silicone models: Imaging and manipulation. Neurol Med Chir (Tokyo) 2005;45:567–573. doi: 10.2176/nmc.45.567. [DOI] [PubMed] [Google Scholar]
- 3.Dayal R, Faries PL, Lin SC, et al. Computer simulation as a component of catheter-based training. J Vasc Surg. 2004;40:1112–1117. doi: 10.1016/j.jvs.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Frosen J, Marjamaa J, Myllarniemi M, et al. Contribution of mural and bone marrow-derived neointimal cells to thrombus organization and wall remodeling in a microsurgical murine saccular aneurysm model. Neurosurgery. 2006;58:936–944. doi: 10.1227/01.NEU.0000210260.55124.A4. [DOI] [PubMed] [Google Scholar]
- 5.Graves VB, Strother CM, Pratington CR, et al. Flow dynamics of lateral carotid artery aneurysms and their effects on coils and balloon: an experimental study in dogs. Am J Neuroradiol. 1992;13:189–196. [PMC free article] [PubMed] [Google Scholar]
- 6.Strother CM, Graves VB, Rappe A, et al. Aneurysm hemodynamics: an experimental study. Am J Neuroradiol. 1992;13:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- 7.Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. J Neurosurg. 1991;75(Part 1: Electrochemical basis, technique, and experimental results):1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 8.Massoud TF, Ji C, Guglielmi G, et al. Experimental models of bifurcation and terminal aneurysms: construction techiniques in swine. Am J Neuroradiol. 1994;15:938–944. [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan ES, Heilman CB, Roth PA, et al. Endovascular packing of carotid bifurcation aneurysm with polyester fiber-coated platinum coils in a rabbit model. Am J Neuroradiol. 1993;14:323–333. [PMC free article] [PubMed] [Google Scholar]
- 10.Spetzger U, Reul J, Wies J, et al. Endovascular coil embolization of microsurgically produced experimental bifurcation aneurysms in rabbits. Surg Neurol. 1998;49:491–494. doi: 10.1016/s0090-3019(96)00437-5. [DOI] [PubMed] [Google Scholar]
- 11.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology. 1999;213:223–228. doi: 10.1148/radiology.213.1.r99oc15223. [DOI] [PubMed] [Google Scholar]
- 12.Raymond J, Salazkin I, Metcalfe A, et al. Lingual artery bifurcation aneurysms for training and evaluation of neurovascular devices. Am J Neuroradiol. 2004;25:1387–1390. [PMC free article] [PubMed] [Google Scholar]
- 13.Grunwald IQ, Romeike B, Eymann R, et al. An experimental aneurysm model: a training model for neurointerventionalists. Interv Neuroradiol. 2006;12:17–24. doi: 10.1177/159101990601200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsumoto T, Terada T, Yamaga H, et al. Coil embolization training using a rabbit saccular aneurysm model. Interv Neuroradiol. 2006;12(Suppl 1):57–60. doi: 10.1177/15910199060120S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouzeghrane F, Naggara O, Kallmes DF, et al. In vivo experimental intracranial aneurysm models: a systematic review. Am J Neuroradiol. 2010;31:418–423. doi: 10.3174/ajnr.A1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenstein A, Song JK, Tsumoto T, et al. Treatment of experimental aneurysms with an embolic-containing device and liquid embolic agent: feasibility and angiographic and histological results. Neurosurgery. 2009;64:367–373. doi: 10.1227/01.NEU.0000335790.91413.64. [DOI] [PubMed] [Google Scholar]